Department of Chemistry
CHE606
Dr. Nikita Matsunaga
 |
Department of Chemistry CHE606 Dr. Nikita Matsunaga |
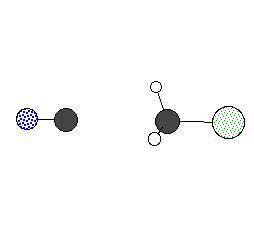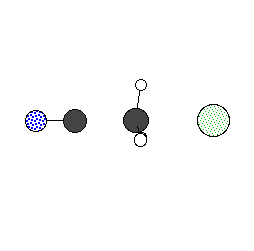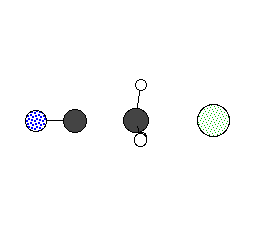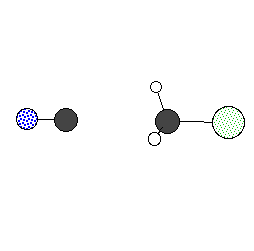
Following animation shows the nuclear
configuration along the reaction coordinate, the minimum
energy path, of an SN2 reaction. A chloride ion approaches
cyanomethane from the opposite end of the NC-C bond. As we
have learned in an introductory organic course, the transition state
structure assumes nearly planar configuration with the central carbon
and hydrogens. Eventually the reaction leads
to the formation of chloromethane and cyanide ion separates.
The bond formation or cleavage in the animation is arbitrary.
Remember that a bond is not a switch!
The following graph shows the potential energy profile corresponding to the nuclear configurations shown in the animation above. The energetics are calculated at the RHF/STO-3G level of theory. The forward reaction barrier is about 50 kcal/mol, and the reverse reation barrier is about 15 kcal/mol.
The following shows the bond lengths calculated for the reactants, products, and transition state.
| Parameter | Bond Length |
| CN | 1.154 |
| C-CN | 1.486 |
| C-H | 1.088 |
| Parameter | Bond Length |
| CN | 1.159 |
| C-CN | 2.093 |
| C-Cl | 2.069 |
| C-H | 1.081 |
| Parameter | Bond Length |
| C-Cl | 1.787 |
| C-H | 1.088 |