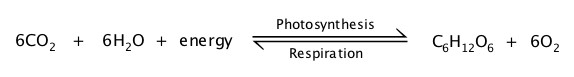
In general, carbohydrates contains C, H and O, as the name implies, and they are made of sugars. Carbohydrates have either ketone or aldehyde that are highly substituted with hydroxyl (OH) groups. One unit of such molecule is the so-called monosaccharide, single sugar molecule. When two sugar molecules are bound together, it is called disaccharide, for which table sugar is typical.
Monosaccharides
Monosaccharides is a simplest form of sugar containing typically 3 to 6 carbons. They include aldehyde containing sugar, called aldose and ketone containing sugar, called ketose. The number of C in sugar molecule gives names:| Name | # of C |
|---|---|
| Triose | 3 |
| Tetrose | 4 |
| Pentose | 5 |
| Hexose | 6 |
| Heptose | 7 |
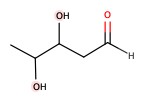
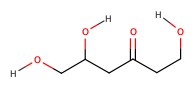
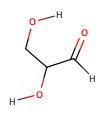
Example: Classify the following sugars:
| a) | b) | c) | d) |
 |
 |
 |
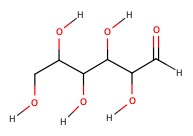 |
a) 5-carbon chain, and aldehyde. Therefore, aldopentose.
b) 6-carbon chain, and ketone. Therefore, ketohexose.
c) 3-carbon, aldehyde. aldotriose.
d) 6-carbon, aldehyde. aldohexose.
Stereoisomers
When carbon has four single bonds and the molecule with the same substituents, is not superimosible, then the carbon on the molecule is said to be chiral. If a molecule possesses a chiral C, there should be a molecule with a connection that is the mirror image of each other, and they are said to be enantiomers.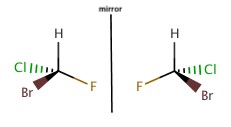
Stereoisomers are the molecules with the same chemical formula and having the same fucntional groups or attachments, but differ in 3-D orientation of the attachemnts.
Enantiomers have different optical properties, more specifically one enatiomer polarized plane-polarized light into one direction and other enantiomer polarized to another direction.
Molecules with More than One Chiral Carbon Atom
Sugar molecules have a lot of chiral carbons! The maximum number of stereoisomers is given by 2n, where n = number of chiral carbons.
One of the most important sugar in our body is glucose. Glucose is one of the 16 stereoisomers stemming from the chemical formula C6H12O6. The structure of glucose is drawn, below, such a way that the backbone of the charbon chain is vertical, and having non-chiral carbons at the top and bottom of the drawing.
Example: Can you identify the chiral centers of the following molecules?
| a) | b) | c) |
 |
 |
 |
Look for a carbon with four different substitutions.
Click below to see the answer.
| a) | b) | c) |
 |
No chiral center in this molecule |  |
Many sugar molecules are chiral. Glucose, fructose and mannose are all chiral compounds. In what is called Fischer projections, depending on the -OH substituents, we give D-sugar designation and L-sugar designation.
The D-sugar occurs when the -OH group attached to the chiral center farthest from the C=O, points to the right.
The L-sugar occurs when the -OH group attached to the chiral center farthest from the C=O, points to the left.
The following is an example of L-sugar.
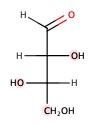
Important Monosaccharides and Monosacchrides Derivatives
Glucose is one of the most important carbohydrates in our body. Glucose is a aldohexose, as shown below with is Fischer projection and the numbering scheme for monosaccharides. Also shown is a fructose.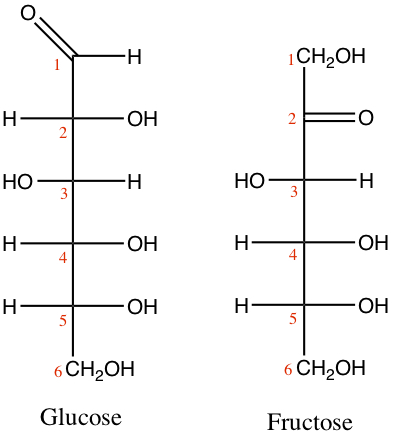
Rxns of Monosaccharides
Sugar molecules, containing either ketone or aldehyde group, that give a positive result in the Benedict's test (Cu2+ is reduced) == Reducing Sugar. For example, D-fructose can be converted in the presence of base to either D-glucose or D-mannose, and vise versa. All three sugars are reducing sugars.
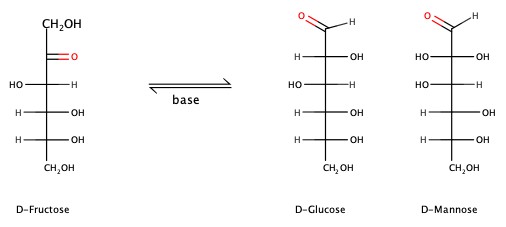
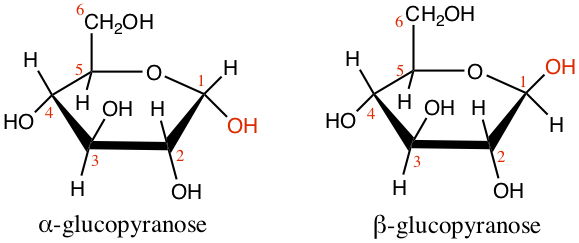
Cyclic Monosaccharides
Less than 0.1% of glucose is in the form of open-chain as we have seen above. Most of the glucose is in the ring form, called pyranose. The ring is formed by the reaction of aldehyde and alcohol, as we have seen in Chapter 9, to form hemiacetal. The D-glucose, shown below, had internal hemiacetal formation by bonding Carbon 1, which used to be aldehyde C and the alcohol group on Carbon 5, producing H2O.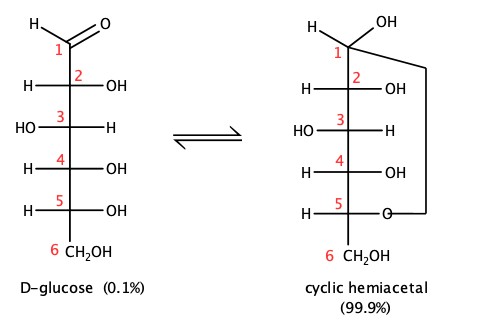
Oligosaccharides
Disaccharide is made by forming a glycosidic bond between two monosaccharides. For example, maltose, produced in dried germinated grain such as barley, is formed by connecting two glucose molecules.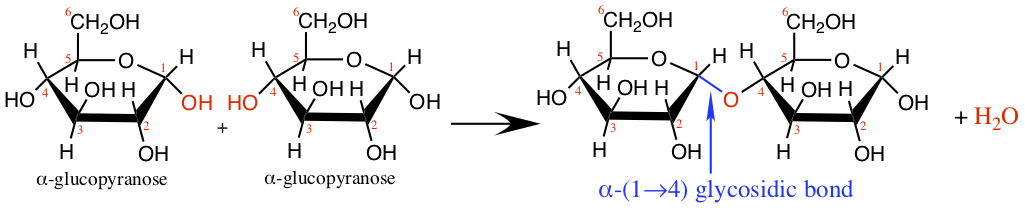
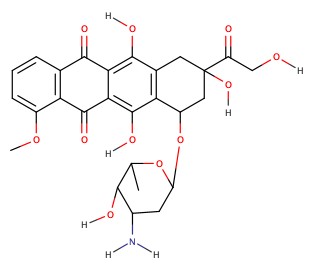
Doxorubicin is an anticancer agent, and the main action is to insert itself between the two rows of the DNA base pairs so that the DNA enzymes can not do its job to replicate the DNA in the cancer cells. Water solubility is achieved by having aminoglucose-like strutcture shown below.
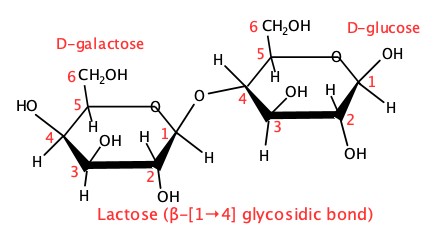
There are many many oligosaccharides. Shown below are the representative of what we encounter in out daily lives.
First one is a lactose. Lactose is produced in milk of mammals. It is a disaccharide, consisting of D-galactose and D-glucose. The glycosidic bond is of β-(1→4) type.
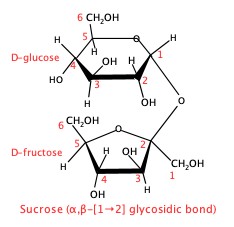
Sucrose is table sugar. It is composed of D-fructose and D-glucose, bonded by α,β-(1→2) gylcosidic bond.
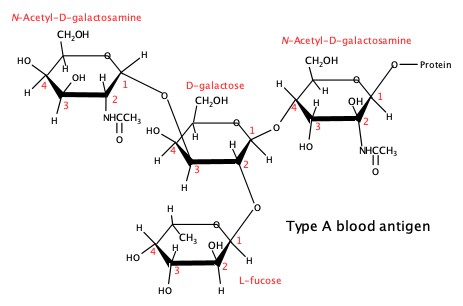
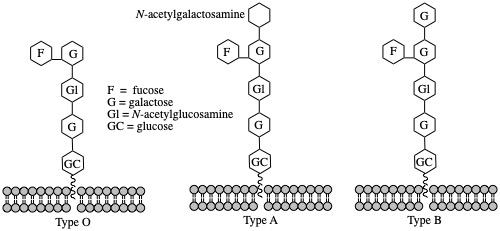
Oligosaccharides are also very important in blood chemistry. The following is the trisaccharide that is responsible for the immune response in mixing different blood types.
Type B blood: Replace the N-acetyl-D-galactosamine residue at the upper left with D-galactose.
Type O blood: Missing the N-acetyl-D-galactosamine residue at the upper left.
Polysaccharides
Polysaccharides are the molecules with very large molar mass by linking making sugars. Polysaccharides are polymers made of individual monomeric sugars.
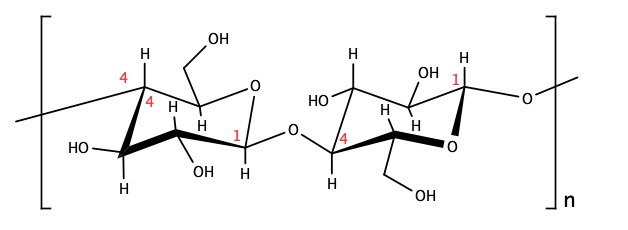
Cellulose made by plant for the cell wall, is a polymer of D-glucose with the β(1→4) glycosidic bond to form the chain. The length of the chain is in the order of hundreds to thousands. In the picture below, the structure in the square-braket is the basic unit of the cellulose and n is the number of repeating units. As mentioned already, the value of n is anywhere from hundreds to thousands.
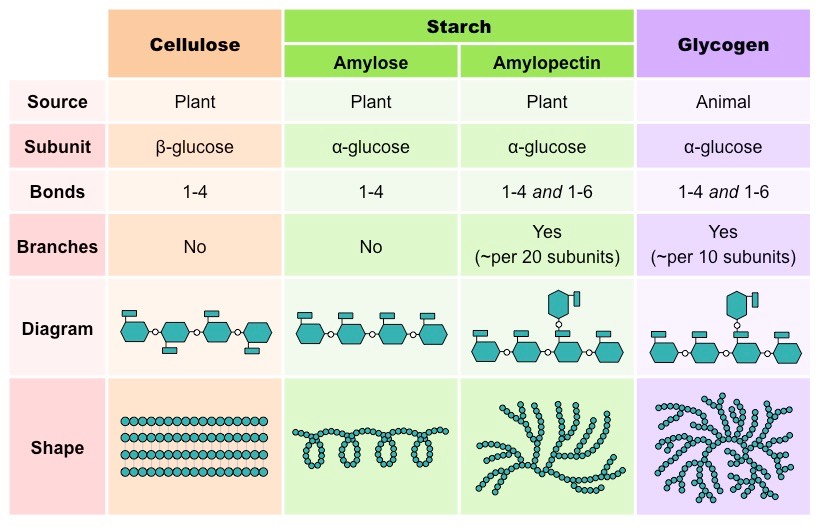
There are many polysaccharides in nature. Below is the cartoon version of four different polysaccharides that we are familiar with.
Got it from Creative Proteomics