Fatty Acids
Fatty acid contains hydrocarbon chains and carboxylic acid group, as shown below.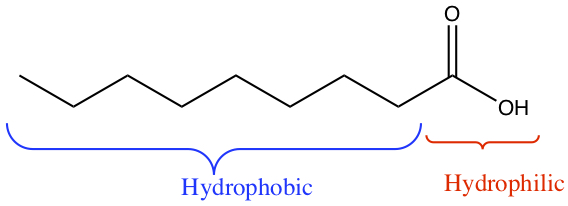
I use canola oil for cooking. The label says the following:
| Fat | # of g |
|---|---|
| saturated fat | 1 |
| trans fatty acid | 0 |
| polyunsaturated fatty acid | 3.5 |
| monounsaturated fatty acid | 9 |
| Total | 14 g (1 serving) |
Saturated fatty acid has no dbouble bonds in the hydrocarbon part of the fatty acid, while unsaturated fatty acid has one or more double bonds found in the hydrocarbon chain.
Usually, the double bonds in fatty acids assume cis conformations, thus provides a kink in the chain. For example, oleic acid is 18-C fatty acid with one double bond with cis conformation.
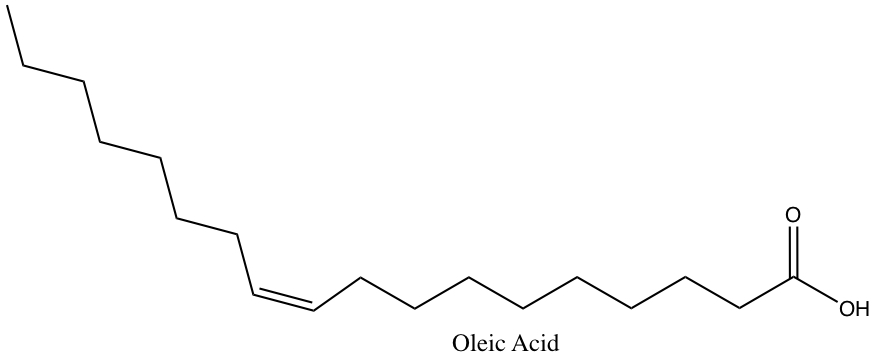
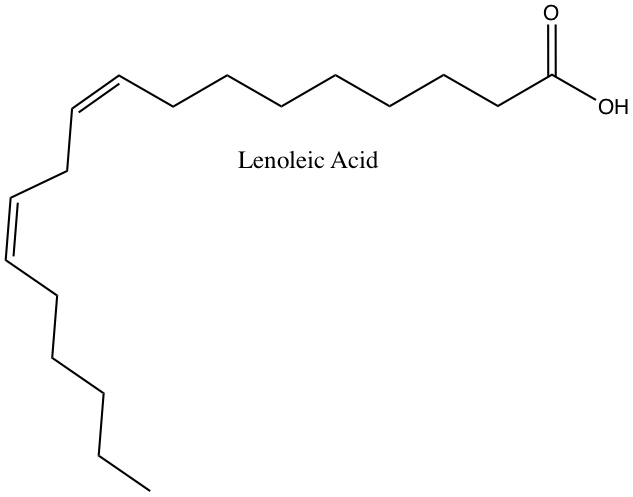
In the table below, fatty acid contents of total fat in some common cooking oil and material.
| Source | Saturated Fatty Acids(%) |
Unsaturated Fatty Acid(%) |
|---|---|---|
| Lard (pork fat) | 39% | 56% |
| Butter | 63% | 32% |
| Cheese | 64% | 32% |
| Milk (whole) | 62% | 32% |
| Milk (2%) | 62% | 30% |
| Margarine | 16% | 82% |
| Olive oil | 14% | 84% |
| Canola oil | 8% | 92% |
| Peanut oil | 17% | 78% |
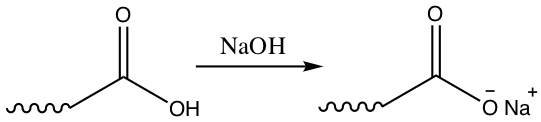
Solubility of fatty acid in water is very low or none. However, if ionize the proton on the carboxylic acid, solubility can increase.
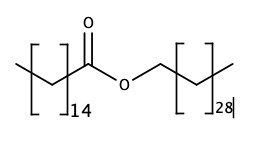
Waxes
Fatty acids + alcohol = Wax (ester).
So, wax is basically an ester with long hydrocarbons on both sides of the ester group. We have already seen the reaction of carboxylic acid and alcohol in the presence of H+ produces ester.
Beeswax used in candles has the following structure.
Triglycerides
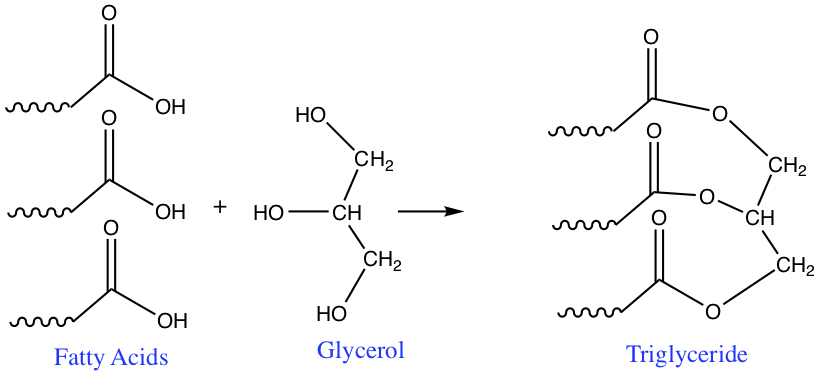
The compounds known as triglycerides are the most common fat. They are contained in food such as butter. Triglycerides are energy storage molecule. The excess amount of caloric intake from food would be converted to triglycerides and it is stored in adipose cell. Fatty acid + Glycerol → Triglycerides
Triglycerides are esters produced from some fatty acids and specific alcohol, glycerol, which is a 3-C triol (3 hydroxyl groups attached).
Triglycerides are energy source. Adipose cell (adipocyte) stores fat in the form of triglycerides. The energy contents of triglycerides are higher than carbohydrates and protein.
Triglycerides > Carbohydrates > Protein
Oxidation spoils unsaturated fats. Unsaturated fat can undergo reaction with O2 and produces small organic molecules, often aldehyde and carboxylic acid. You may have tasted the funny tasting oil that is rather old.
You can make soap from triglycerides by saponification (well, just hydrolysis using NaOH or KOH) to produce glycerol and water soluble salt of fatty acid. The following facts are interesting!
- When NaOH is used, soap becomes solid.
- When KOH is used, soap becomes liquid.
Olestra
Since triglycerides have more energy contents in the per gram basis, a low-calorie alternative was sought out. The compound is even approved as a food additive by the U.S. Food and Drug Administration (FDA). Olestra's structure is as follows: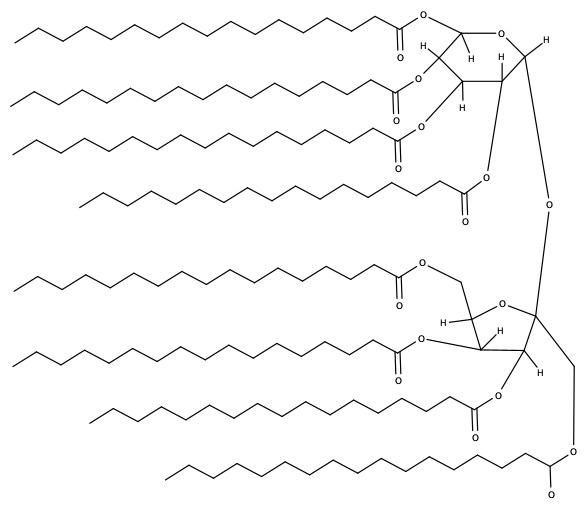
Phospholipids and Glycolipids
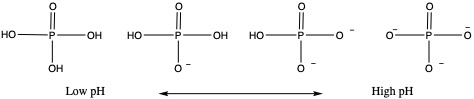
As the name implied that phospholipid is compound made of lipid with phosphate group (PO4 unit) attached. Phospholipids are abundant in biochemistry. They are the main component of cell memebrane.What's unique about phospholipid is that, depending on pH, protonation level of phosphate group changes, as shown below.
Cell membrane in animals and plants are made of glycerophospholipids. It consists of fatty acid, glycerol, and phosphate groups, and the structure is shown below:
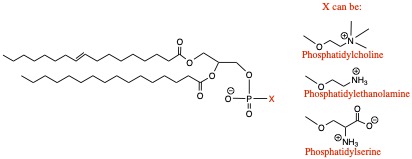
- The most common glycerophospholipid is phosphatidylcholine found in the cell membranes of plants and animals. Phosphatidylcholoine is also a component of lipoprotein for transporting lipids in the blood streams.
- The second most commond phospholipid is phosphatidylethanolamine and is found in the cell membrane. It is important in active transport, for example, of lactose across membrane.
- Less common, but still important is phosphatidylserine , which possesses serine amino acid. It is commonly found in myelin sheath that wrapps around a nerve cell in the brain tissue.
- Human red blood cell membrane contains 21 different phosphatidylcholine, differing in fatty acid parts of the molecule!
Steroids
Steroids are a type of lipids that possess fused rings. The general feature of steroids are as follows.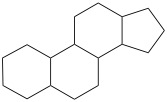
The most familiar one is cholesterol, as shown below.
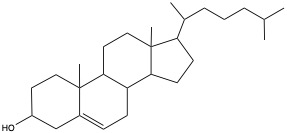
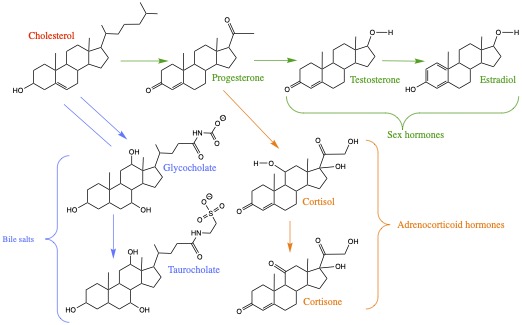
Cholesterol can be converted to progesterone, female reproductive hormone, which in turn can be converted to testesterone and estradiol. Also, cholesterol can be converted to bile salts, such as glycocholate and taurocholate to help emulsify to aid digestion. Cortizone and its modifications are used as anti-inflammatory medicine. These compounds are all biosynthesized in your body from cholesterol.
Anabolic Steroids
One can artificially increase the production of muscle cells, often illegally enhancing the athletic performance. Anabolic hormones is used to stimulate protein synthesis and muscle building. Look at these anabolic steroids below; their structures are closely related to testosterone.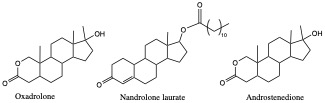
Eicosanoids
The "eico-" part of eicosanoids come from a latin word for 20. For example, icosahedron has 20 faces. Arachidonic acid and other 20-C polysaturated fatty acids make up a class of lipid called Eicosanoids. They are used by our body as cell signaling molecules. Some of the physiological functions of eicosanoids include:- mounting or inhibiting inflammation, allergy, fever and other immune responses
- regulation of the abortion of pregnancy and normal childbirth
- contribution to the perception of pain
- regulation of cell growth
- controlling of blood pressure
- modulation of the regional flow of blood to tissues
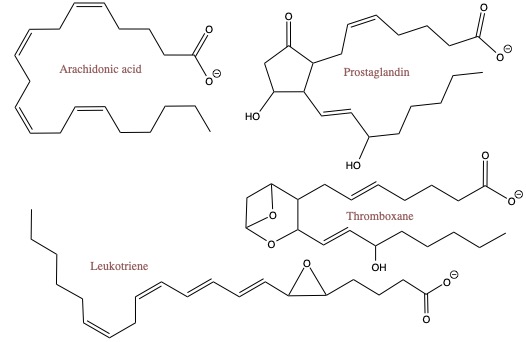
Arachidonic acid is converted to prostaglandin via enzymatic reactions. Aspirin and ibuprofen, pain relievers, disrrupts the enzymatic process to alleviate the pain.
Thromboxanes is responsible in blood clotting. Leukotrienes are responsible for muscle contractions in the lungs, and are linked to asthma attacks.
Membranes
Biological membrane separates self from the outside. As we have been examining cell membrane, seemingly from the begining of the semester, is consist of mainly phospholipids, but also glycolipids, cholesterols and proteins are involved.
We have already seen how the phospholipids look like. Shown below is a model of phospholipid and its cartoon version.
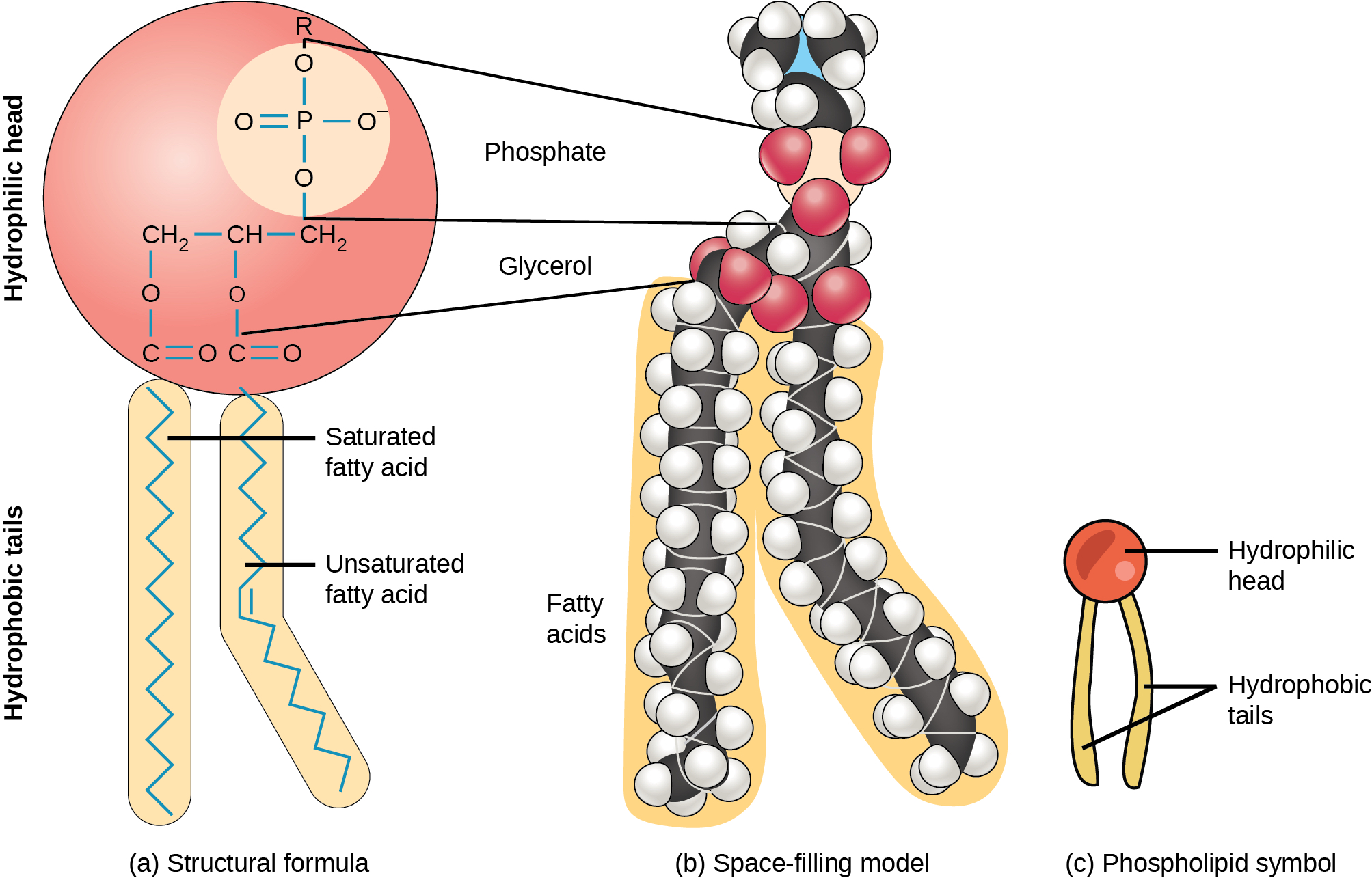
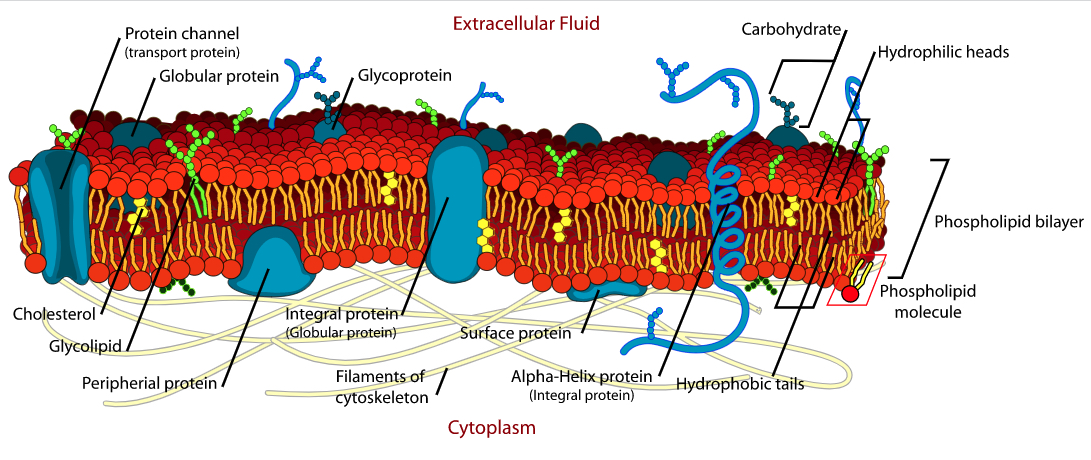
I've shown you the following video before, but it is instructive to look at it again!