- Structure
- Catalysis
- Transport
- Immunity
Amino Acids
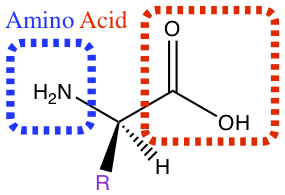
Amino acid contains amine group and carboxylic acid functional groups which are separated by a carbon with side chains, represented by R in the figure below. Different R gives different amino acid. There are 20 naturally occuring amino acids foun in nature among plants and animals.
Classification
Here, we classify amino acids into polarity as well as acidity. These characteristics are dependent upon the substitution of the R group, written in purple in the picture above. The table below shows the R group and their properties.| R1) | Name | Symbol2) | Polarity | Acidity3) |
|---|---|---|---|---|
| H | Glycine | gly G | nonpolar | |
| CH3 | Alanine | ala A | nonpolar | |
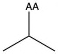 | Valine | val V | nonpolar | |
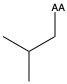 | Leucine | leu L | nonpolar | |
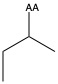 | Isoleucine | ile L | nonpolar | |
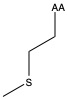 | Methionine | met M | nonpolar | |
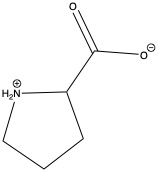 | Proline | pro P | nonpolar | |
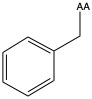 | Phenylalanine | phe F | nonpolar | |
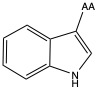 | Triptophan | trp W | nonpolar | |
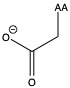 | Aspartic acid | asp D | polar | acidic |
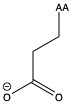 | Glutamic acid | glu E | polar | acidic |
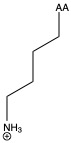 | Lysine | lys K | polar | basic |
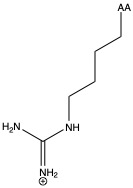 | Arginine | arg R | polar | basic |
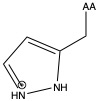 | Histidine | his H | polar | basic |
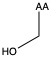 | Serine | ser S | polar | neutral |
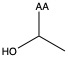 | Threonine | thr T | polar | neutral |
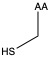 | Cysteine | cys C | polar | neutral |
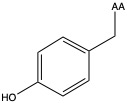 | Tyrosine | tyr Y | polar | neutral |
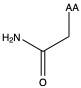 | Asparagine | asn N | polar | neutral |
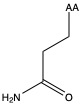 | Glutamine | gln Q | polar | neutral |
1)In the first column, R refers to the side group. The letters "AA" represents where the R group attaches to the amino acid backbone.
2) "Symbol" in the table above refers to either 3-letter or 1-letter symbol commonly used by biochemists.
3)Acidity is assigned for polar amino acids only.
Stereochemisitry
The stereochemistry of amino acid is represented by the Fischer projection in the following way:
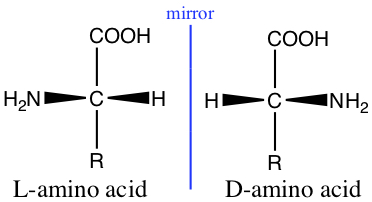
Glycine, with R = H, has no stereo chemistry, but all other naturally occuring amino acids have stereochemistry.
The Peptide Bonds
As has been discussed in the previous chapters, carboxylic acid can react with amine to form amide. Amino acid can form dipeptide by forming amide bond between two amino acids. The amide bond between two amino acids is called peptide bond.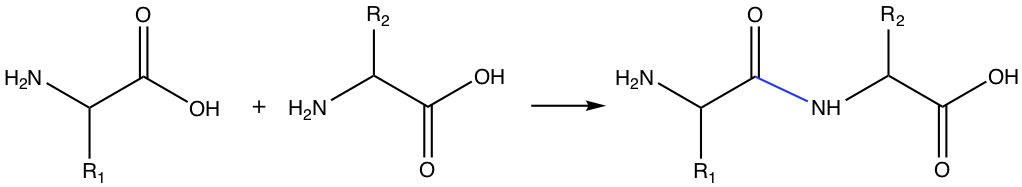
In the figure above, the product is called dipeptide, since the product has two distinct amino acid. If there are several amino acids form a chain of amino acid by forming peptide bonds, it is called oligopeptide. When the chain length becomes large, the chain is a protein.
The directionality of peptides are often discussed in terms of N-terminus and C-terminus, where N-terminus is the side of the amino acid chain that has the terminal amino acid has amine group, and where C-terminus has carboxylic acid at the end of the chain. The following figure illustrates the designation for the tetrapeptide.
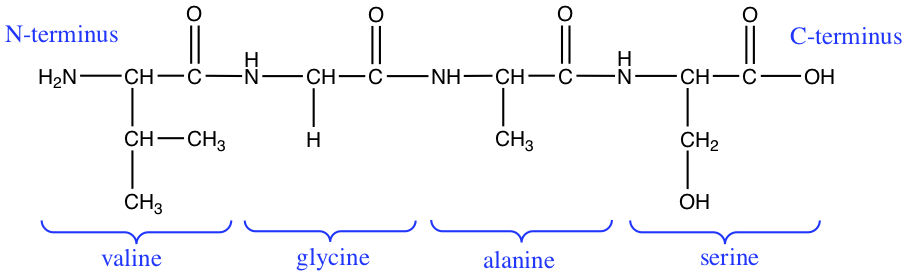
Peptides, Proteins, and pH
Depending on the pH of the solution, the amino acids and their chains have different protonation levels. As can be seen below, at low pH, with abundant H+, the amine group is protonated. At high pH, the concentration of OH- is high, thus takes the H off of the carboxylic acid. At pH = 7 or physiological pH, the amine group is protonated, and carboxylic acid is deprotonated. The situation where + charge and - charge present in one molecule is called zweitterion.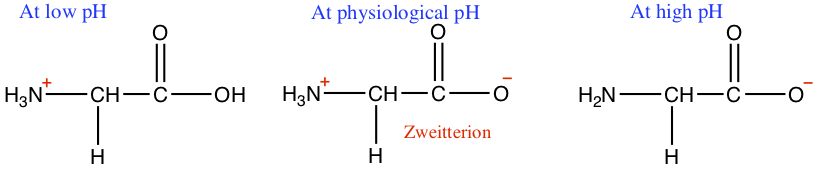
Protein Structures
In order to function properly, amino acid chain has to fold into three-dimensional form, typically globular protein for which most of the catalytic and motor protein belong to, and fibrous protein found in structural proteins.
Primary Structure
Primary structure is simply the chain of amino acid sequence. The length of sequence varies in length. For example, hemoglobin has about 300 residues over two different chains.
Secondary Structure
Secondary structure is a partially folded form of sequence. There are two types: α-helix and β-sheet.
The α-helix, as the name implies, form a helical structure, and between turns one might observe a number of hydrogen bonding occurs to keep the helical strucuture in place.
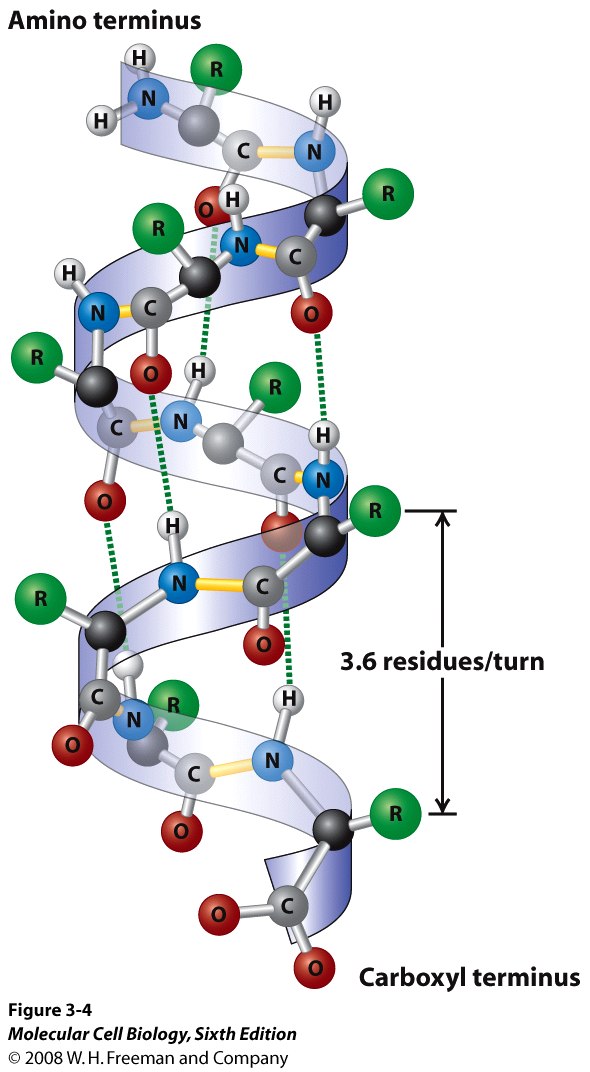
The β-sheet is a relatively flappy sheet-like structure, held also by hydrogen bonding.
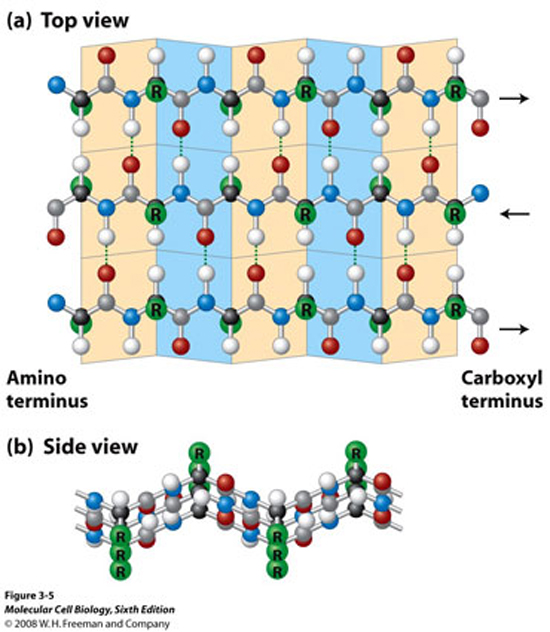
Tertiary Structure
Tertiary structure is a completely folded and functional protein. In general, the secondary structures are put together in some globular shape, and the protein might contain smaller organic molecules to perform some function. For example, rhodopsin found in the rod cell of retina responds to specific wave length of light, is shown below, represented in the ribbon model.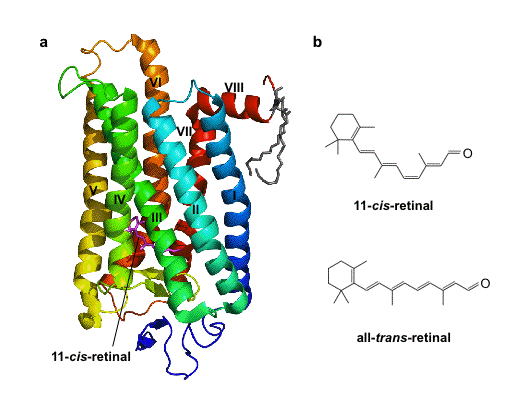
Let's see this in 3D. Here you can see the 3D structure.
Quaternary Structure
Although, some proteins are functional with the tertiary structure, but many proteins form quaternary structure. The quaternary structure is a oligomeric form of tertiary structured proteins.
Insulin has a trimer of dimeric structured protein. Each of the three dimers are a dimeric form of polypeptide chains.
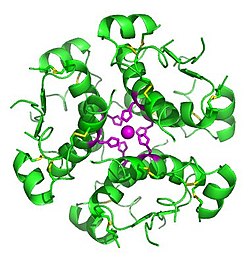
Denaturation
When protein loses its function due to either heat or pH change is called denaturation. Upon heating or change of pH will bring permanent change in the three-dimensional strucuture of protein. Only the native structure of protein is functional and other forms are not functional.
Enzymes
Enzymes catalyze reactions. Nearly all reactions in our body are enzymatic reactions. Since it is catalysis, the reaction speed is much faster than those without enzymes. Enzymes are named with -ase. So for exampl, if the enzyme carries out esterification of some compound, then you may see something like esterase.
Pictorially, enzymatic reaction looks like the following, and the initial binding of substrate to the enzyme is an equilibrium process.
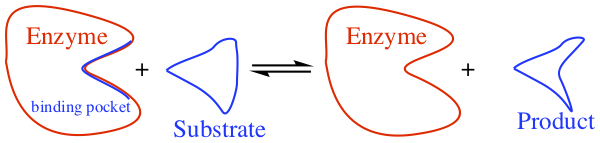
There might be a need for cofactors, inorganic ions for enzymes to carry out the reactions. In some cases, coenzyme is required for the reaction to be carried out. For example, NADH (nicotinamide adenine dinucleotide) can be used as a source for redox centers in the redox based rxn enzymes.
Control of Enzymes--Catalyzed Rxns
Many of pharmaceuticals target enzyme inhibition. The line of thought is that if one can interrupt one of biochemical pathways that is so important for the particular bacteria/virus/cancer/others, the causal agent can not survive, hence this leads to the cure. So, this is a very big game in large pharmaceutical companies and research done at many universities and national labs alike.
The affinity of substrate and/or inhibitor toward enzyme is given by Michaelis-Menten binding constant, since the binding of substrate to enzyme is an equilibrium process we can write down the expression for the equilibrium constant. We call this equilibrium constant, Michaelis-Menten binding constant.
Inhibitors can be classified in many ways. One of the requirements is that the inhibitors must show high affinity toward binding to the enzyme, meaning that the Michaelis-Menten constant is much much larger than 1.
Severe Acure Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) aka COVID-19
Timely topic, related to this chapter dealing with proteins, is of course COVID-19. From what we talked about this semester, much of the structure of the virus as well as its life cycle may become understandable. The following picture came from here and shows the structure of the coronavirus that has been causing the pandemic.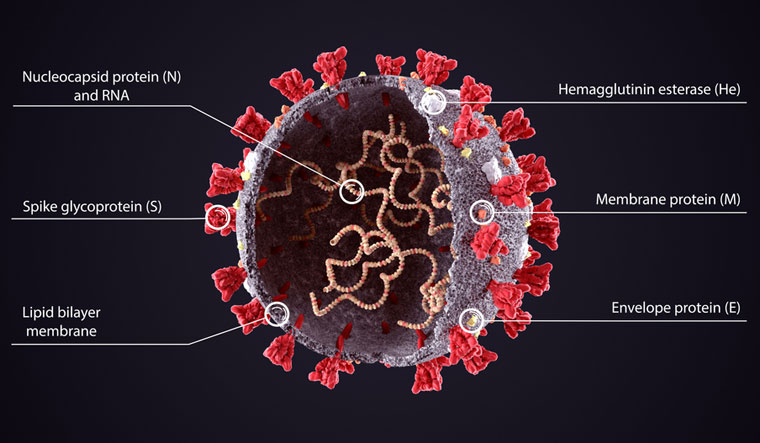
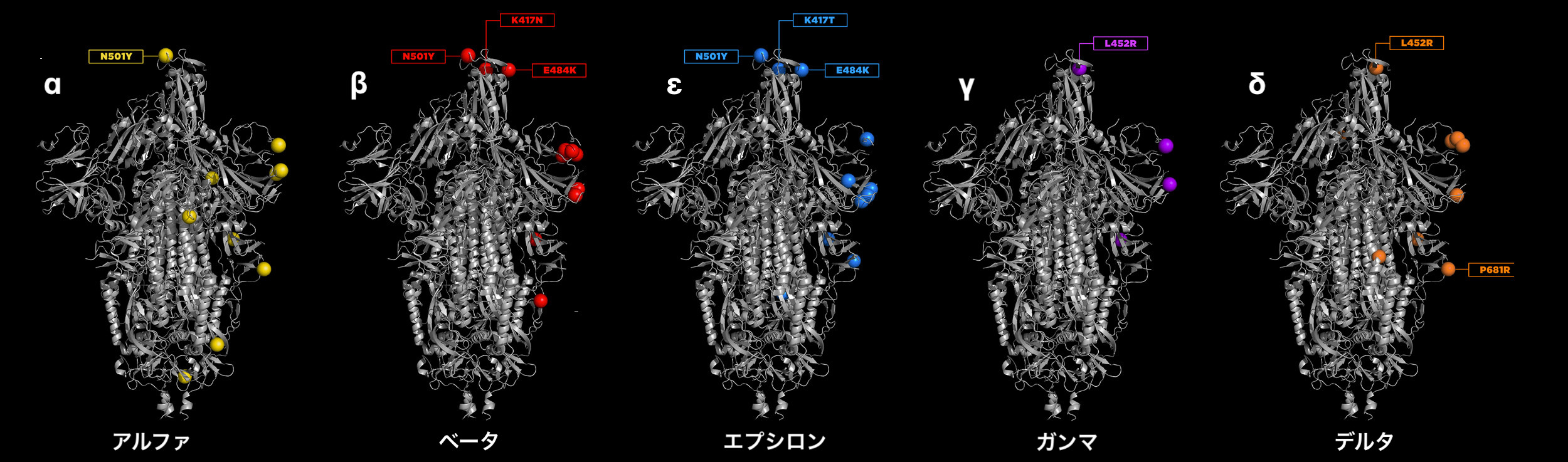
Now that we hear the news about ο variant of COVID-19, let's understand different variants. Each of the variants differs in its sequence of the primary chain of amino acids. The mutation is very subtle; they only differ few places in the sequence. You can see the mutations in the varints up to the δ variant.
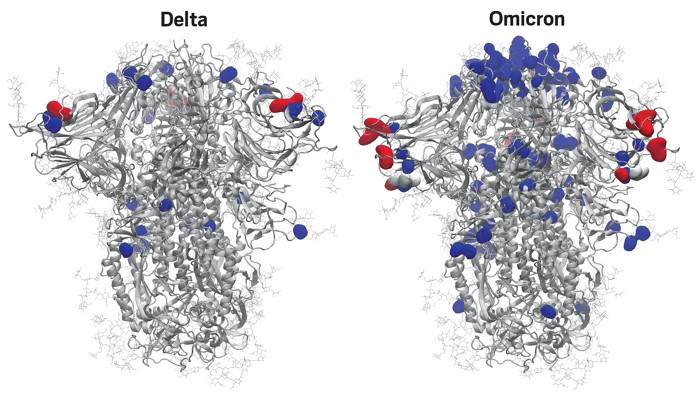
Latest!!! Just obtained a picture of ο (omicron) variant's mutation, in comparison with the δ variant. This came from From C&E News
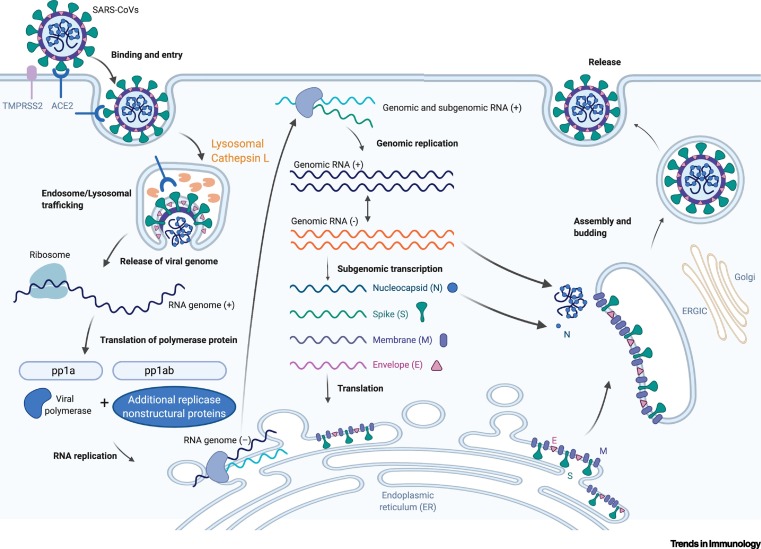
The following picture represents the life cycle of COVID-19, taken from here
Videos!
Below are some videos that give you more pictorial representations of what we talked about. They are no so much as to give you details, but are for you to get the feeling of how proteins are utilized in our body.
The first one is in the category of strucutral proteins. Collagen is structural protein found in skin and connective tissues.
Below is a video of enzymatic reactions.
Immunoprotein, antibody, is explained in the following video.
The last one, but not least interesting!, is the how the molecular level understanding of mitosis process! I think this is one of the most amazing videos I've seen in biochemistry! The motorprotein is obviously hot research area. Enjoy!
... in the End
... and in the end, I want to show you the biochemical pathways that all living things on Earth share. These are almost all the chemical reactions happen in our body every second of our lives. I hope that you can appreciate how complicated they are, and simultaneously many of the molecules and the reactions become little more familiar to you at this juncture.
 .
.