Atoms
Atom = fundamental unit from which matter is constructed.
Atomic Structure
Atom looks like the following:
The following table shows the comparison of subatomic particles in atoms.
| Subatomic Particle | Mass (g) | Mass (amu) | Charge | Location in atom |
|---|---|---|---|---|
| Proton | 1.6726 x 10-24 | 1.0073 | +1 | Nucleus |
| Neutron | 1.6750 x 10-24 | 1.0087 | 0 | Nucleus |
| Electron | 9.110 x 10-28 | 5.486 x 10-4 | -1 | Outside of nucleus |
On average, where would the electron be, if I place the baseball-proton on the table in this lecture room?
Here, you have the answer!
Atom is electrically neutral. It means that the number of protons and the number of electrons are equal.
Elements
Element is a group of atoms that contains only one type of atom.There are little over 100 elements exist. Aside from their names, we assign atomic symbol, either one or two-letter symbol.
For example, carbon's atomic symbol is C, H for hydrogen, O for oxygen, etc.
Trace Elements
Trace elements are elements that the body needs only small quantities to function properly.For example, iron in our body is found in hemoglobin and iron binds oxygen molecule to proceed respiration.
| Element | Biochemical Significance |
|---|---|
| Iodine (I) | Production of thyroid hormones |
| Iron (Fe) | Found in hemoglobin of red blood cells to carry oxygen |
| Zinc (Zn) | Growth, wound healing, smell and taste |
| Copper (Cu) | Formation of hemoglobin |
| Manganese (Mn) | Metabolism |
| Cobalt (Co) | prodcution of red blood cells |
| Selenium (Se) | Acts as antioxydant |
Atomic Number and Mass Number
Atom can be represented by the following manner. The one on left is what's appeared in the Periodic Table, and the one on right is another representation of element.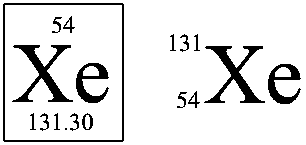
In the representation on left is from the Periodic Table. The number on top is the atomic number. The number shown at bottom is the average mass of the element in the atomic mass unit (amu). The average is over all naturally occuring isotopes.
Example: Give the number of protons, neutrons and electrons in the following atoms. a) b) c)
When the atoms are presented in the format, the subscript is the atomic number (Z), and the superscript is the mass number (A). So, the number of protons is Z, and the number of neutrons is A - Z. The number of electrons are the same as Z in all cases, since there is no charge.
a) 17, 35-17 = 18, and 17, respectively.
b) 18, 23, 18
c) 23, 27, 23
Isotopes
Atoms in an element that have different number of neutrons.For example, carbon has several isotopes in nature. The most abundant one is , but it is also found that and in nature in small quantity.
Interesting article in HealthLink on stable isotopes and drug testing. You should read it!
Periodic Table
Each row = periodEach column = group
Each cell contains: Atomic Symbol, Atomic Number, and Average Mass.
The elements can be classified as Metals, Semimetals, and Nonmetals.
Groups
Groups are numbered from left to right as 1 to 18. scheme. Some of the names associated with the groups are:
Periods
Elements in the same row is said to be in the same period.
The Mole
Mole is a numbering scheme for atoms or molecules.Since there are little over 600 000 000 000 000 000 000 000 water molecules in merely 18 mL!
To be more exact, there are 6.02 X 1023 in 12.011 g of carbon. The number, 6.02 X 1023 is called Avogadro's number. Then, it is said that 12.011 g contains 1 mole (mol) of carbon.
Once it is defined through carbon, we can relate carbon to other elements through the Periodic Table.
The Mole and Mass (molar mass!)
The average mass we found on the Periodic Table in amu, is actuall the number of grams of substance in 1 mole.calculations of mol <--> g <--> #
Example: How many moles are there in 2.0 g of carbon?
Example: How many carbon atoms are there in 0.000 000 000 10 g carbon?
Even though, the gram quantity is still so small, there are so many C atoms!
Example: How many g are there in 3.0 mol of carbon?
The Arrangement of Electrons
Quantum mechanics is the law of nature at this realm. What comes out of quantum mechanics is the properties of atoms and molecules.Shell models
Valence Electrons
The chemistry occurs at outer rim of the atoms and molecules. The region is called valence shell, and the electron in the valence shell is valence electrons.
Electron Dot Structures
Each group on the Periodic Table can be assigned for a number of valence electrons.
| 1A | 2A | 3A | 4A | 5A | 6A | 7A | 8A |
|---|---|---|---|---|---|---|---|
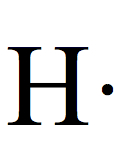 |
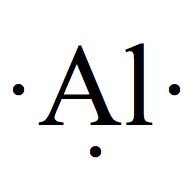 |
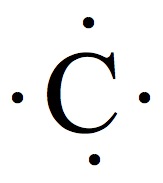 |
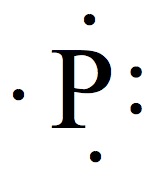 |
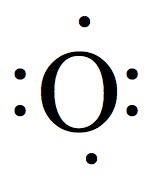 |
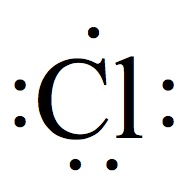 |
Radioactive Isotopes
People of 1800's started to realize that alchemy, which had been practiced from antiquity to the medieval period, to make ordinary metal into gold, didn't work by using chemical techniques and knowledge that they had accumulated.
But, in the late 1800's, we began to see some nuclei of atoms transmutated into other atoms via means of radioactivity.
Raidoactivity is the emission of high energy radiation or particle come from atomic nucleus.
Common Forms of Nuclear Radiation
The radiation come off of radioactive material can be classified as follows:Nuclear Equation
→ +
→ +
→ +
→ +
Here you can actually see the decay α decay of polonium-210 in a DIY cloud chamber.
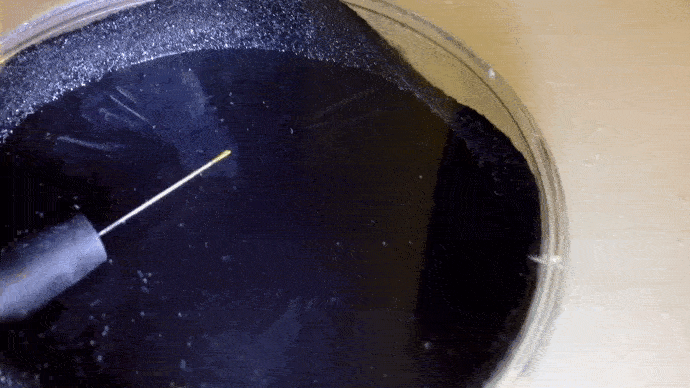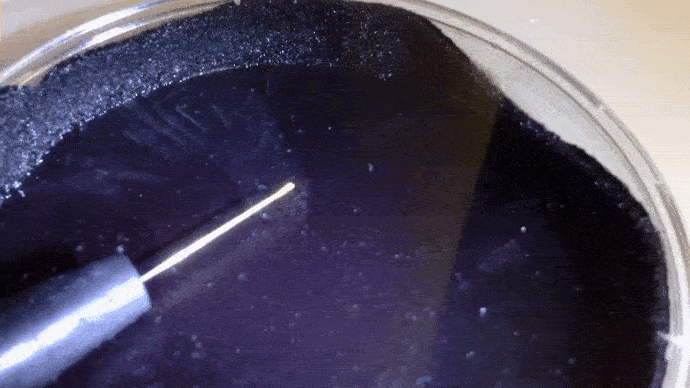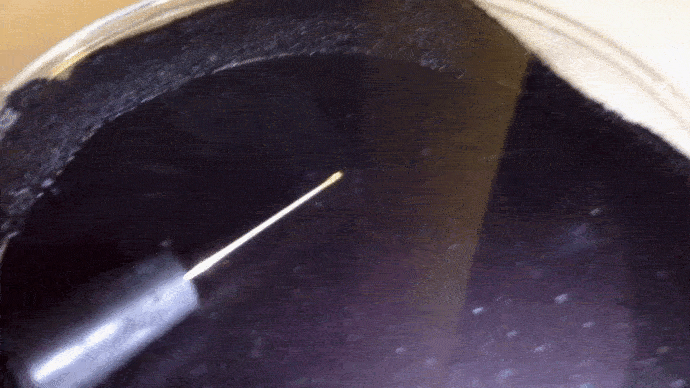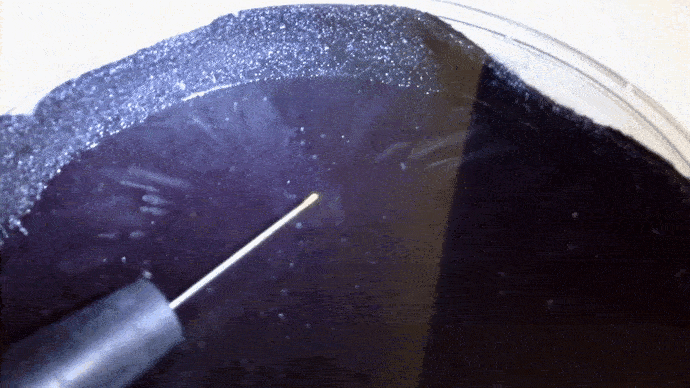
Example: Identify the missing product in the following. a) b)
a) In order to balance nuclear equation, the total of Z on the reactants are the same as the one the products, as well as the total of A on the reactants are teh same as the one on the products.
So, the reactant Z = 19 and A = 40, while the product side has Z = 18 and A = 40. Therefore the product side is missing 1 plus charge, but the mass is the same. It means that the missing particle is a positron. Thus,
b) The similar argument is applied. The left-hand side has Z = 15 and A = 32. On the right-hand side, Z = 1 and A = 0, thus the missing element must have Z = 14 and A = 32. Look up periodic table for Z = 14, and find it as Si.
Radioisotopes in Medicine
Dosage Units
Disintegration per second (dps)
One way to understand nuclear chemistry is to know how many nulei decay within a unit time. The nuclear decay (disintegration) is measured in the unit of curie (Ci), named after Marie Curie, is given by:
Energy related unit (dose)
The energy absorbed by an object is measured in rad (radiation absorbed dose)Different radiation affects biological system in different way, and if such effect is taken into account, we use a unit of rem.
| QF | Which particles |
|---|---|
| 1 | X-rays, gamma rays, beta particles, and positrons |
| 10 | neutrons, high-energy protons |
| 20 | α particles, fragments of fission reactions |
The following diagram shows natural and medical source of radiation.
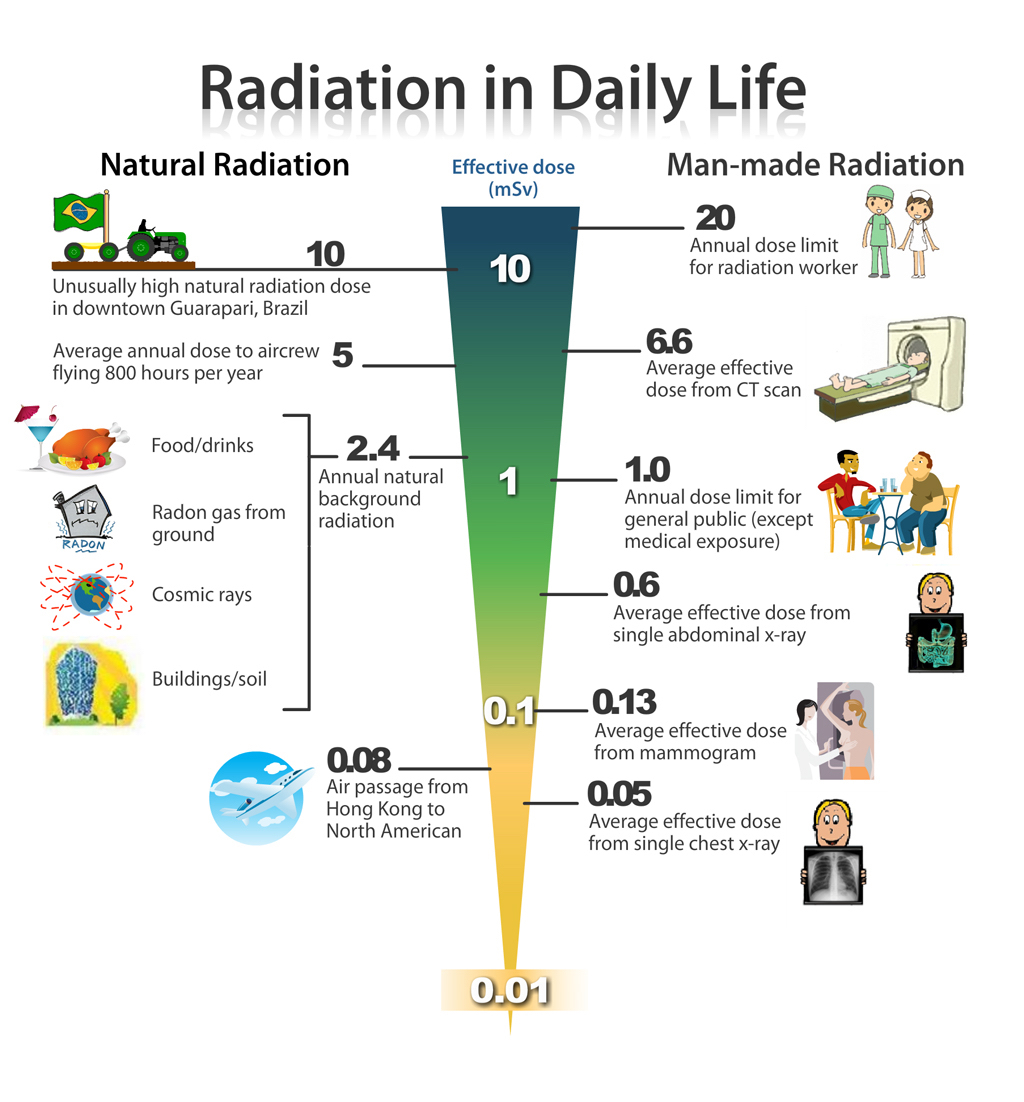
Example: 2.99 Your body contains about 0.1μCi of naturally occuring potassium-40. This number of μCi corresponds to how many disintegrations per a) second? b) minute?
a) Since 1 Ci = 3.7 x 1010 dps, we have
b) for per minute we write the result of above as, Then, we convert it by,
Example: 2.97 Exposure to 50 rem of radiation can cause nausea
and a temporary drop in the white blood cesll count.
a) Convert this value to seiverts.
b) Convert this value into rads, assuing thata the source of radioactivity
is an α emitter.
c) Convert this value into rads, assuing thata the source of radioactivity
is an β emitter.
a) You can do a dimensional analysis, as below.
b) We know that rem = rad x QF, where QF for α particle is 20. Since we know the rem number, we have to rearrange the equation and solve for rad.
c)Similar calculation as above, but QF for β particle is 1, then
Real-lif Example: Explosion of Fukushima Nuclear Powerplant Between March 11, 2011, Fukushima Daiichi Nuclear Powerplant was damaged by tsunami, large tidal wave generated by earth quake. Within few days and few days apart, three of the six nuclear reactors had the so-called "hydrogen explosion," due to meltdown of the uranium fuel. Although the reactors are damanged by the explosion, the reactors were not bleached, meaning that there is no opening on the reactors to spew out radioactive materials uncontrollably. However, in order to reduce the pressure inside the reactors, they vented the highly radioactive contaminated gas to the atmosphere.
Because of the release of radioactive gas, the surroundin area had been contaminated. About 60 miles south of the exploded nuclear power plant, there is another nuclear power plant, which had the web-based monitoring post. The following diagram is the one I got for March 16, 2011.
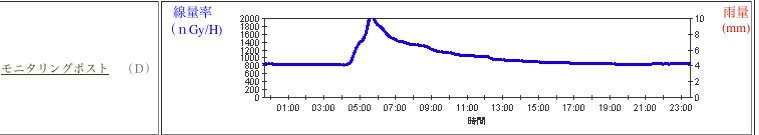
Since you are exposed for two hours, you can do the conversion: Therefore, it is similar to the natural radiation you get as you travel to Hong Kong or Australia from NYC.
The video below is an interesting video showing different kinds of radiation in our lives.
Half Life
Time required for half of the radioactive atoms to decay.For example, has half life of 20 min. So, if you start with 10 g of , after 20 min., you have: OK. Fair enough, what about if you start with 10 g again, after 1 hr how many g does it have left?
Diagnosis and Therapy
Many radioisotopes are used in medicine.
Positron Emission Tomography (PET). This device monitors the glucose metabolism by adding radioactive fluorine to glucose. Glucose is said to be labeled by radioactive fluorine. Following is the nuclear reaction of radioactive F-18.
Computed Tomography
Computers are fully used in today's medical devices using radiation and others. More specifically, Computed tomography (CT) or cmoputer-aided tomography (CAT) is an integral part of diagnostic tools. Tomography is an imaging technique to make 2D slice and 3D imaging using various devices, such as PET, X-ray scan (generally referred to CT scan), and MRI (radiowave monitored in the high magnetic field).
Here are the differences in images:
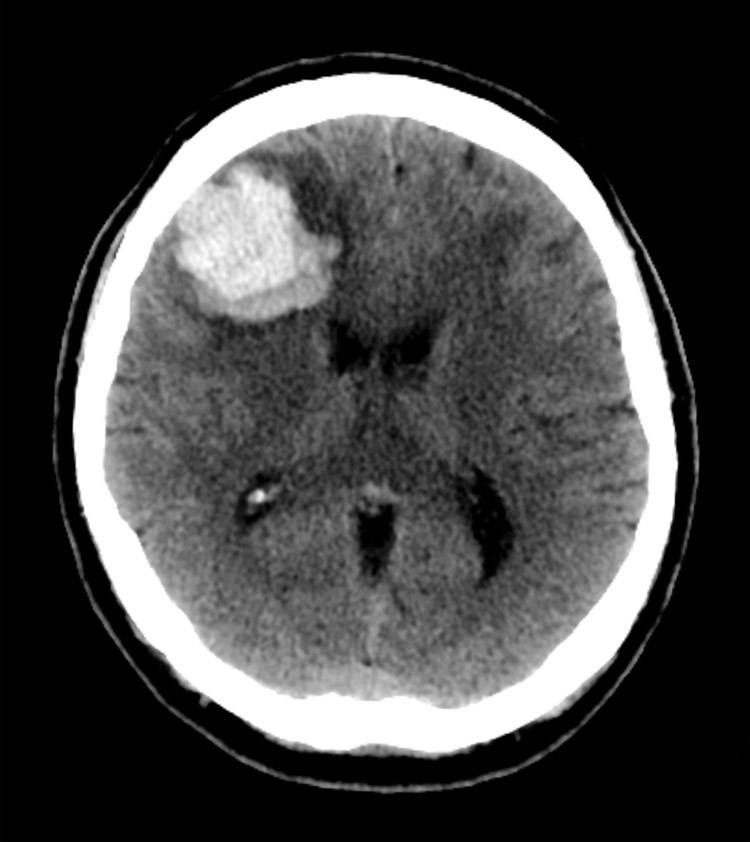 |
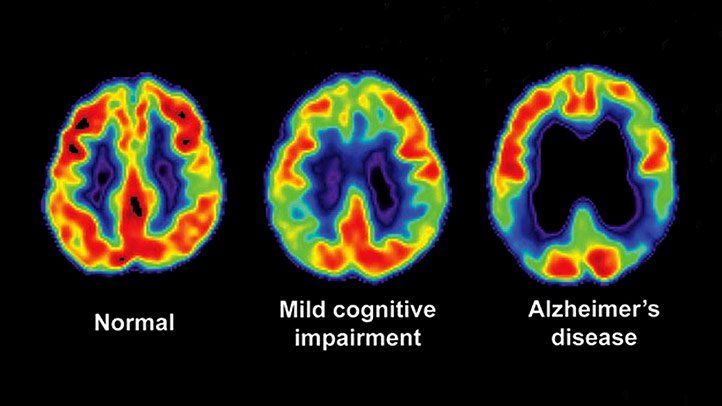 |
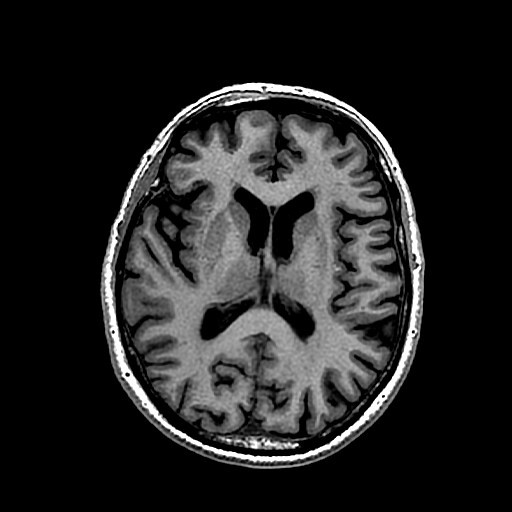 |
| CAT | PET | MRI |
| Radioisotope | Utility | Mode of decay | Half-life |
|---|---|---|---|
| Detection of blood vessel obstruction | γ | 15 hr | |
| Spleen imaging; detecting gastrointestinal disorders | e- capture | 27.7 d | |
| Detection of bone marrow disorders | β | 44.5 d | |
| Detection of bone marrow disorders | positron | 8.2 hr | |
| Treating cancer (Figure 2.31) | β | 5.27 yr | |
| Treating lymphomas; whole-body tumor scans | e- capture | 78.3 hr | |
| Pancreas scans | e- capture | 120 d | |
| Measuring cardiac output | e+ | 32.8 d | |
| Bone scans | β | 64.8 d | |
| Treating prostate and brain cancer | e- capture | 59.5 d | |
| Treating and detecting thyroid disorders | γ | 15 | |
| Detecting lung disorders | β and γ | 5.2 d | |
| Treating cancer (Figure 2.31) | β and γ | 30.2 y | |
| Bone and tumor scans | e- capture | 9.6 d | |
| Pain relief for bone, postate, and breast cancer | β | 3.8 d | |
| Internal radiation therapy | β and γ | 74 d | |
| Brain scan | β | 64 d | |
| Targeted α therapy | β | 45.6 m | |
| Targeted α therapy | α | 9.9 d |