Structural Formula
Drawing Lewis Structures
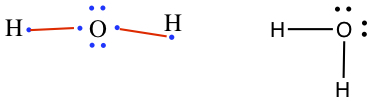
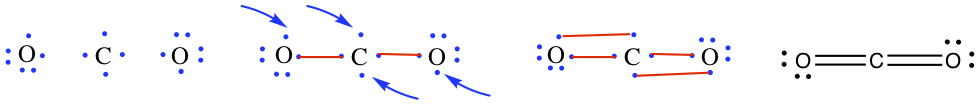
Don't deviate from:Example: Draw Lewis structures for a) H2O, b) CH4, c) CO2, and d) C2H6O.
a) Draw all atoms with valence electrons, and connect unpaired electrons. Then, redraw.
b) Same procedure, but notice that there are double bonds involved.
Example: Draw Lewis structures for a) H2O,
Skeltal Structure
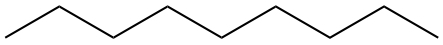
Since it is cumbersome to write in all the atoms in a molecule, we most often use skeltal structure to represent Lewis structure. A line represents C-C bond, and C-H bonds are eliminated from the drawing, but it is understood that all necessary C-H bonds are present.
| Name | Condensed Formula | Lewis Structure | Skeltal Structure |
|---|---|---|---|
| Ethane | CH3CH3 | 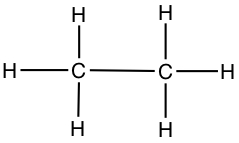 |
 |
| Propane | CH3CH2CH3 | 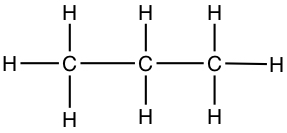 |
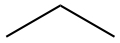 |
| Butane | CH3CH2CH2CH3 | 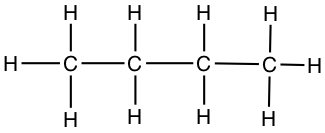 |
 |
| Pentane | CH3CH2CH2CH2CH3 | 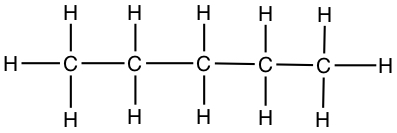 |
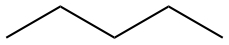 |
| Hexane | CH3CH2CH2CH2CH2CH3 | 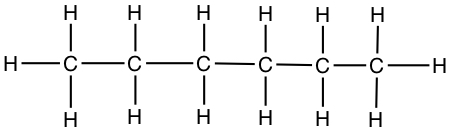 |
 |
| Septane | CH3CH2CH2CH2CH2CH2CH3 | 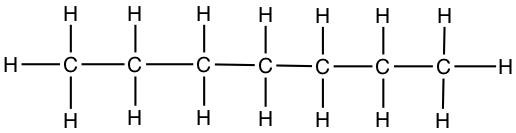 |
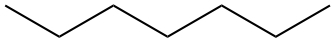 |
| Octane | CH3CH2CH2CH2CH2CH2CH2CH3 | 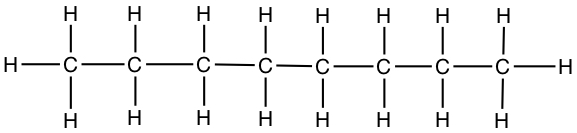 |
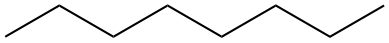 |
| Nonane | CH3CH2CH2CH2CH2CH2CH2CH2CH3 | 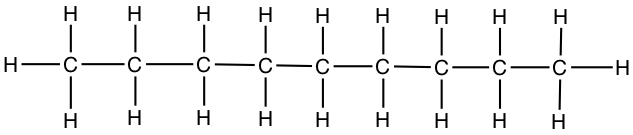 |
 |
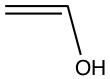
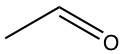
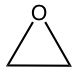
| The following shows different structures arises from the same formula | |||
| C2OH4 | 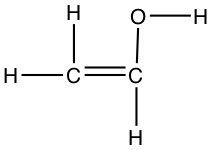 |
 |
|
| C2OH4 | 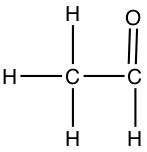 |
 |
|
| C2OH4 | 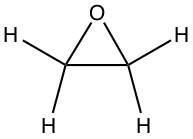 |
 |
|
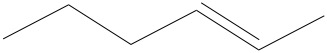
Example: How many carbons and hydrogens are there in
the following compounds: a)
 b)
b)
 c)
c)
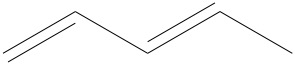 d)
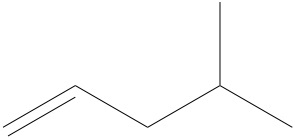
d)
Remember that Carbon can only have four bonds! The lines in the skeltal structure represent a bond between two carbon atoms.
a) By counting the edges that connect lines, you get the number of carbons. Then each of the end carbons get 3 hydrogens, while each of the inner carbons that are bonded to two other carbons get 2 hydrogens. Therefore, the compound (butane) has 4 carbons and 10 hydrogens.
b) There are 6 edges, thus 6 carbons. Whenever one has a double bond, the number of hydrogen is reduced by one hydrogen per carbon. Thus, in this case, there are 12 hydrogens.
c)5 carbons and 11 hydrogens. (Look at the butane, butadiene and butadiyne examples below for the count of hydrogens)
d)6 carbons and 12 hydrogens.
Polar Covalent Bonds, Shape, and Polarity
Ploarity
Not all covalent bonds are created equal!
The two electrons are not shared equally between two atoms. When the electrons are not shared equally, the bond is said to be polar. The polarity of a bond is decided by Electronegativity. Electronegativity is the ability of an atom to attract electron. So, higher the electronegativity, higher the partial negative charge is on the atom within the molecule.
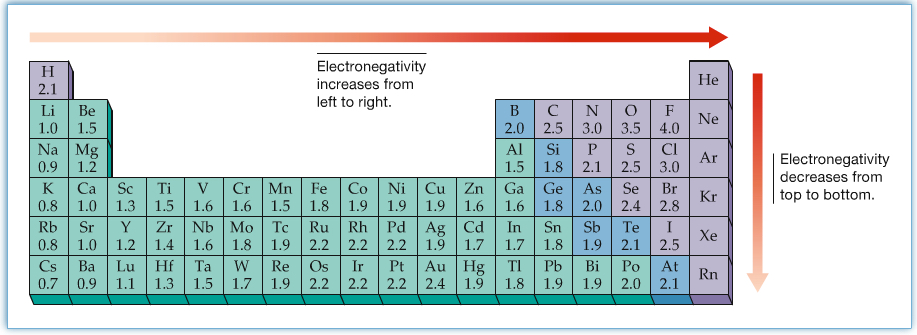
The difference between the electronegativities of two atoms decides how polar the bond is. As indicated in the following figure, you can say that a bond is nonpolar when the difference between two electronegativity of bonded atoms are about 0 to 0.5. If the difference is large and more than 2 or so, then the bond is ionic. The difference values in between, 0.5 to 1.9 or so, are considered to be having polar bonds.
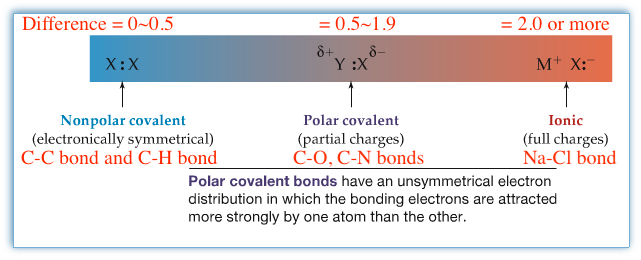
Example:Indicate which bonds are nonpolar, polar, or ionic. If polar, place δ+ or δ-.
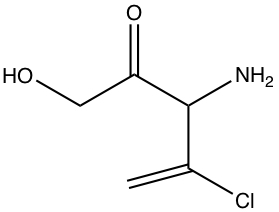
All C-C and C-H bonds are nonpolar. So, all the hetero atoms, referred to non-C and
non-H atoms must be considered. There are four bonds that are polar, indicated by
purple color. For a given polar bond, the partial charges are also given. The bond
between C-Cl is only moderately polar.
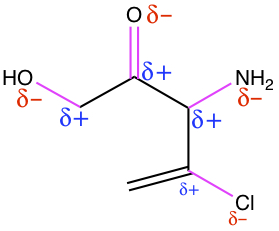
The reason that polarity of bond is important is that organic reaction can to a certain extent be predicted.
Three Dimensional Shape
From above, you know that carbon forms four covalent bonds.| Name | Example | Model | Lewis structure | 3D-drawing |
|---|---|---|---|---|
| Tetrahedral | Methane, CH4 |
|
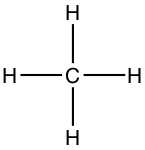 |
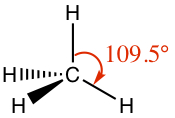 |
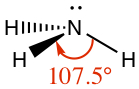
| Pyramidal | Ammonia, NH3 |
|
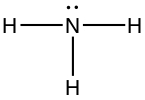 |
 |
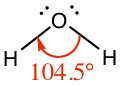
| Bent | Water, H2O |
|
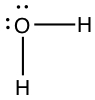 |
 |
| Bond | Geometry | Angle | Example | Details |
|---|---|---|---|---|
| sinle | Tetrahedral | 109.5° |  | 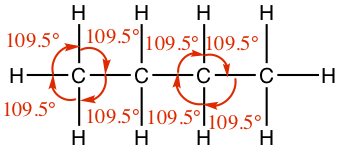 |
| double | Planar | 120° | 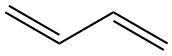 | 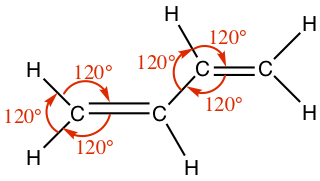 |
| triple | Linear | 180° | 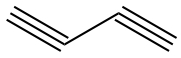 | 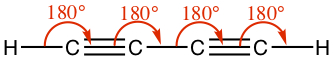 |
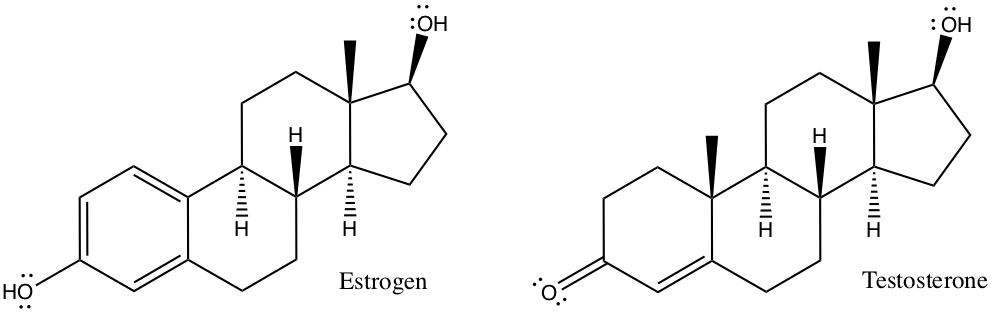
Example:The following figure shows human hormones that are dominant for female and male, respectively. They look quite similar to each other! Identify the geoemtry of each carbon whether it assumes tetrahedral (Td), planar (Pl), or linear (Ln) configuration.
First, the notation used here needs clarification. The solid tapered bonds,
 , are the
bonds pointing toward you out of the plane of the
computer screen, and the dashed tapered bonds,
, are the
bonds pointing toward you out of the plane of the
computer screen, and the dashed tapered bonds,
 , are the bonds pointing
away from you, out of the plane of the screen. At the end of the
bonds there exit carbon atoms, as we have understood from how we
represented skeltal structures. The ones with the hydrogen,
, are the bonds pointing
away from you, out of the plane of the screen. At the end of the
bonds there exit carbon atoms, as we have understood from how we
represented skeltal structures. The ones with the hydrogen,
 and
and
 , have the
same meaning, but there is no carbon there. Instead, there is a
terminal hydrogen. Generally we specify such H due to symmetry of the
righthandedness or lefthandedness of the molecule. This becomes
very important in enzyme (protein) chemistry!
, have the
same meaning, but there is no carbon there. Instead, there is a
terminal hydrogen. Generally we specify such H due to symmetry of the
righthandedness or lefthandedness of the molecule. This becomes
very important in enzyme (protein) chemistry!
From above, all singly bonded C assumes tetrahedral configuration, all doubly bonded C assumes planar configuration, and all triply bonded C assumes linear configuration.
Click below to see the answer.
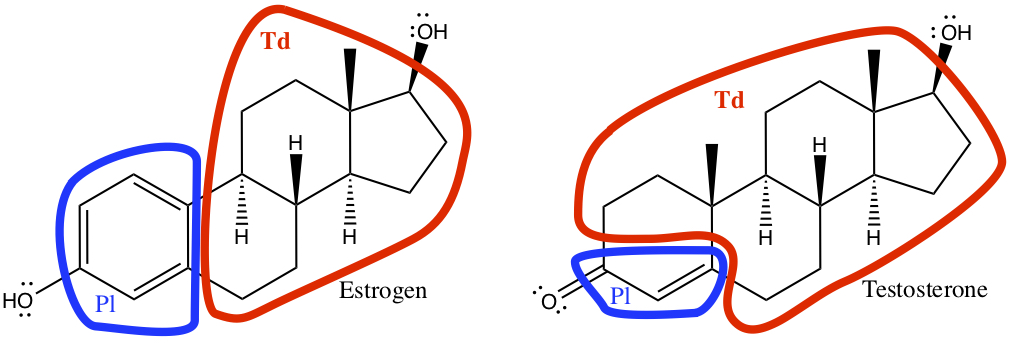
All the carbons in one of the circled areas have the same configurations.
Noncovalent Interactions
Chemical bonds are results of interactions between between atoms in a molecule. There are another level of interactions that have to do with physical processes such as liquid boiling to became gas or cooling liquid become solid. These processes are the result of interaction between molecules, thus we call them, intermolecular forces. Two types:
Noncovalent Interactions Due to Permanent Charges
According to the electronegativity difference, Δχ, between two atoms, we can classify if the molecule has permanent partial charges as we have seen above.Hydrogen bond is a type of interaction that shares hydrogen atom between two molecules. Water is a good example. In the following image, water molecules are connected by hydrogen bonds (in red dashed lines).
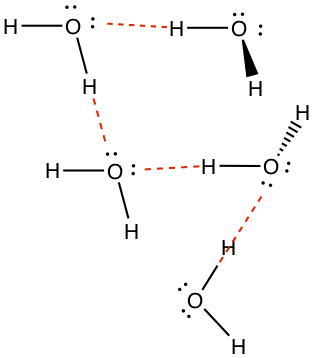
When the small molecule that possesses -OH or -NH2 group often has hydrogen bonding.
Dipole-dipole force is due to the permanent dipole moment from polarity of bonds in the molecule. The following illustrates the interaction between molecules possessing dipolar bonds.
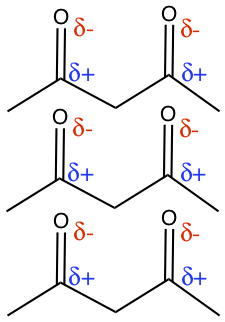
Noncovalent Interactions Due to Temporary Partial Charges
The outermost layer of molecule is covered with electrons. In nonpolar molecules, the situation is the same. So, the molecule is a body of minus charges, represented below as δ-. When the two nonpolar molecules approach each other, the electrons nearest to each molecules start to feel each other; there are repulsion between them because of two minus charges repel each other. If and when molecule B is "softer," then the electrons on molecule B at the side of incoming molecule would go away. Because electrons are missing at the position, the molecule develops positive charges, represented by δ+. So, the positive charge will be attracted by the minus charges of molecule A to form a stable complexation. The intermolecular interaction of this kind is said to possess London force.
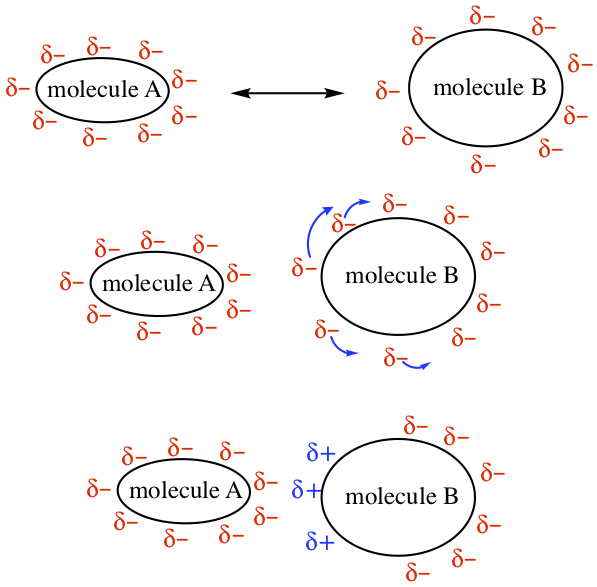
Example:Indicate the type of predominant intermolecular interactions each of the following molecules posssess:
| a) |  |
| b) | 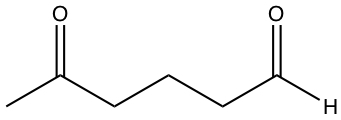 |
| c) | 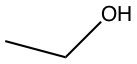 |
| d) | 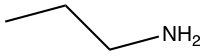 |
| e) | 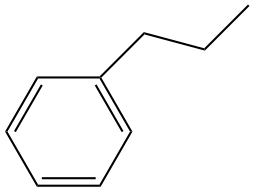 |
a) and e) are hydrocarbons. There is no polarity to bonds. Therefore, only the London forces.
b) has C=O two places within the molecule, therefore dipole-dipole force.
c) and d) -OH and -NH2 substituents are considered to have hydrogen bonding.
Families of Organic Compounds
Functional Group -- A group of atoms in a molecule that has specific chemical properties.
Hydrocarbons
Alkanes

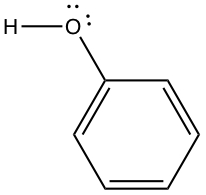
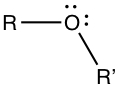
Alcohols, Phenols, and Ethers
Alcohol group contains -OH substituents.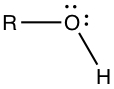
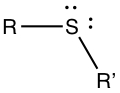
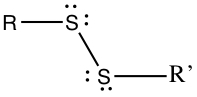
Thiols, Sulfides and Disulfides
Thiol group contains -SH substituents. Again, R is some organic group.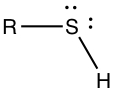
Amines
Amine group contains N in three different arrangements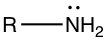 |
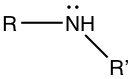 |
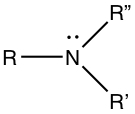 |
| Primary (1°) amine | Secondary (2°) amine | Tertiary (3°) amine |
Alkyl Halides
Halogen such as F, Cl, Br, or I is attached to tetrahedral C.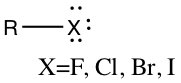
Ketones and Aldehydes
Ketone has C=O substituent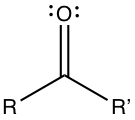
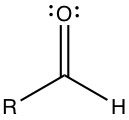
Carboxylic Acids, Esters, and Amides
Carboxylic Acid has -COOH substituent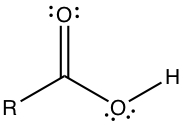
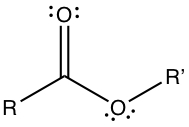
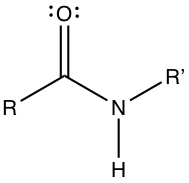
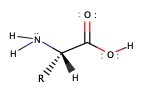
Amino acid ia an important molecule for biochemistry. It is a building block of protein. Because protein acts as structural molecules as well as reaction catalysts. Amino acid has a simple structure, which contains amine group and carboxylic acid group. Below is the structure of amino acid.
Example:Identify functional groups in the following:
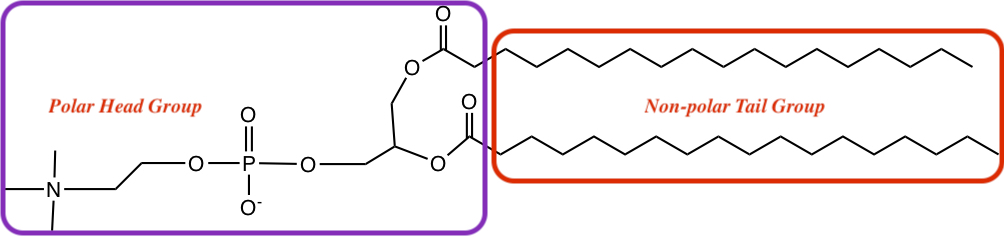
Click below to see the answer.
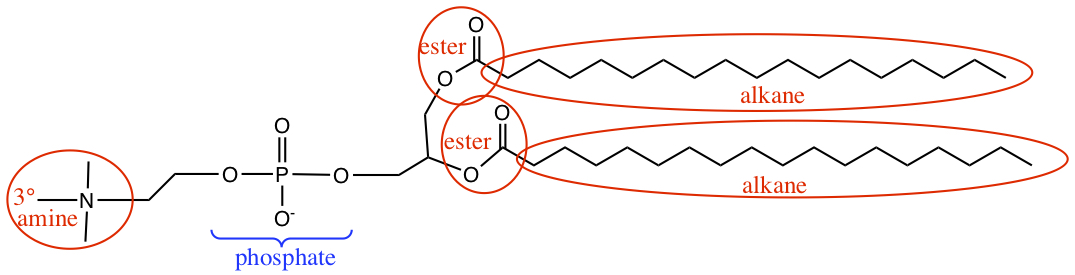
Example:Identify functional groups in the following:
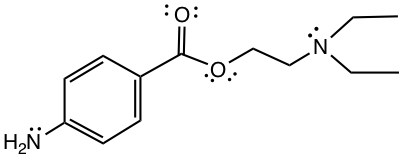
Click below to see the answer.
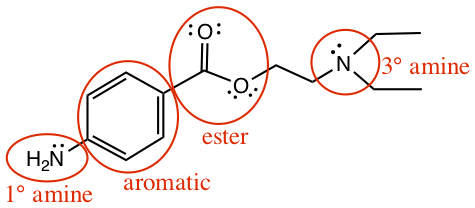
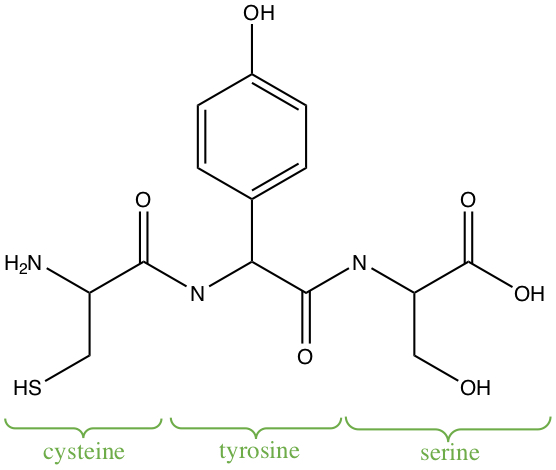
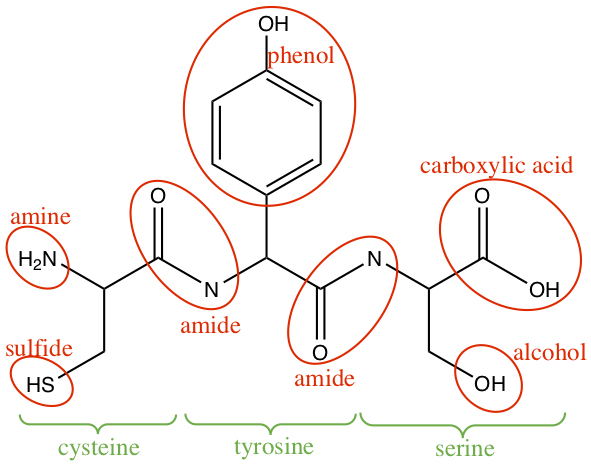
Example:Identify functional groups in the following trimer of amino acids: