Chemical Equations
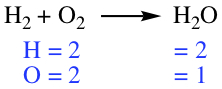
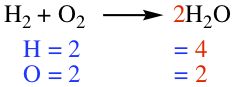
Chemical equation tells how chemical transformation occurs in a reaction. Reactant is separated from the Product by an arrow, as shown below.
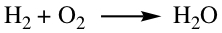
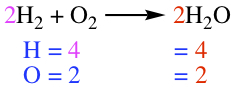
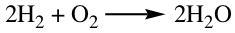 |
Example:Balance the following chemical equations.
a)
Now, I can see that there are four F on the right-hand side, so multiply 4 to HF.
b) Simlar line of thought,
c) This NO2, exhausted out of cars, reacts with oxygen and water causes the acid rain (rain of nitric acid!!). Yikes!
N = 1, O = 5, H = 2 → N =1, O = 3, H = 1
Let's start with multiplying 2 for water
We need four HNO3,
Then, make 4 NO2
Reaction Types
We can classify reactions into four categories:
Synthesis Reaction
Decomposition Reaction
Single Replacement Reaction
Double Replacement Reaction
Reaction Involving Water
The functional groups that are required to undergo reactions that involves water are: alkenes, alcohols, carboxylic acids, and esters. In all cases, we use H+ (proton = meaning we have acid) as a catalyst for the reaction. Catalyst is a facillitator of reaction but not get consumed by the reaction; just a helper to do the job, and leave when it is done.
Mechanistically, the reactions are initiated by a catalytic proton, attracted to the partial negative, δ-, charge of oxygen on oxygen containing functional groups or a double bond of alkene. Subsequently, water molecule is attracted to the positively charged protonated functional group.
Alkanes are not reactive toward water.
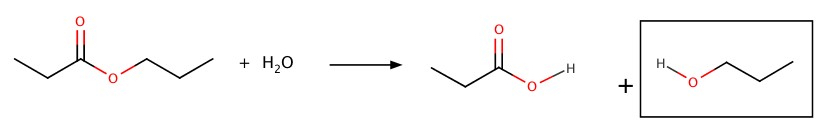
Hydrolysis
Ester is hydrolyzed to produce a carboxylic acid and an alcohol.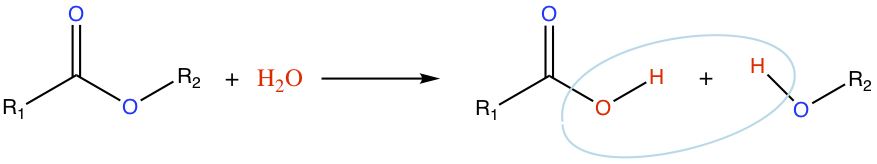
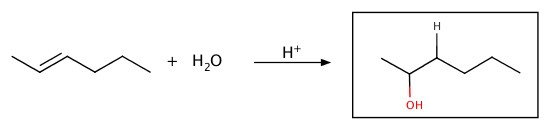
Hydration
Water can be added to alkene to produce an alcohol.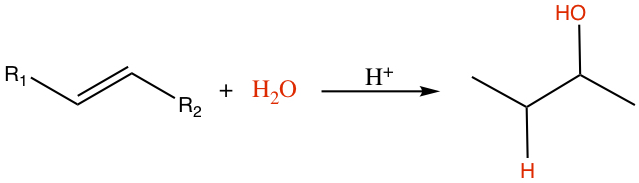
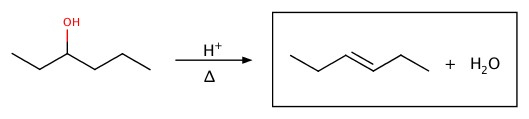
Dehydration
Alcohol can be decomposed to alkene.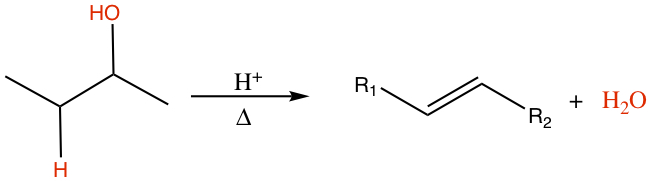
Example:Fill the blank in the following reactions.
a)
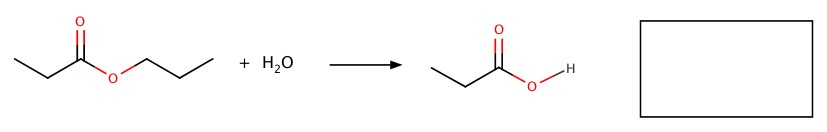
b)
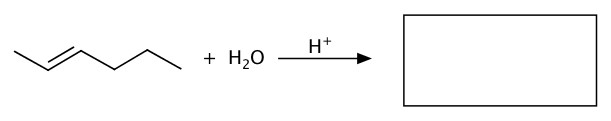
c)
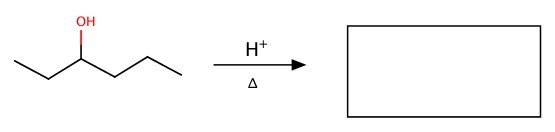
Oxidation-Reduction (Red-Ox)
Reduction = in a chemical process, the compound gains a number of electronsOxidation = in a chemical process, the compound loses a number of electrons
In this situation, we call Cu2+ ion to be oxidizing agent, and Al to be reducing agent.
Combustion as a Red-Ox Rxn
Combustion is a chemical process that add oxygen to carbons of hydrocarbon compounds to produce carbon dioxide and water.
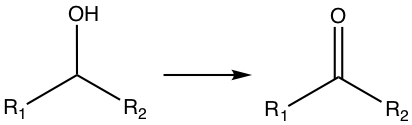
Oxidizing Alcohols and Aldehyde
If the OH is at the terminal carbon, the reaction produces aldehyde.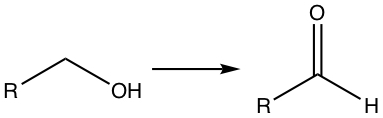
If the OH is in the middle, the reaction produces ketone.
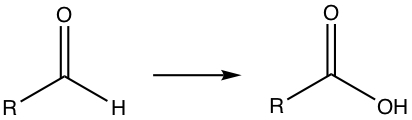
Aldehyde can be converted to carboxylic acid.
Hydrogenation
Platinum metal, Pt, is used as catalyst to hydrogenate alkene, aldehyde and ketone.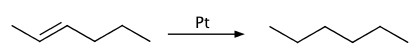
Mole and Mass Relations
In this section we examine the relationship between the masses of molecules involved in a reaction as well as the relationship between the number of moles of molecules in the reaction.
We have looked at molar mass and the mole concept for elements in Chapter 2. We also know how to calculate molar mass of molecule according to Chapter 3. We are now going to relate different species in terms of quantity, either mass or moles, that are involved in the reaction.
Given the balanced chemical equation:
| 2H2 | + | O2 | → | 2H2O | |
| 2 molecules | 1 molecule | 2 molecules | Microscopic View | ||
| 2 mol | 1 mol | 2 mol | Macroscopic View |
Microscopic view == molecular picture
Macroscopic view == quantities that can be measured (like g and mL)
Example:Let's start with simple example. Given that
Since 2 mol H2 yields with 2 mol H2O, it stands to reason that 10 mol H2 produce 10 mol H2O. Let's put this into dimensional analysis. is how you start.
Following the rule of dimensional analysis to cancel the available unit with the denominator of the conversion factor placed inside of the parenthesis, we use the balanced chemical equation as our conversion factor, and that is 2 molH2 = 2 molH2O. Then,
Example:Now, a simlar question, but adding complexity. Given that
Again, we start with 2 mol H2, but this time we are seeking the gram quantity of H2O. So we ask: Since we started with 10 molH2, the first parenthesis is the same as the example above. However, we now seek gH2O, so we need a way to convert gH2O into molH2O, and the g to mol (or mol to g) conversion is accomplished by using the molar mass. The molar mass of water is given as 2 X (1.008 g/mol) + (16.00 g/mol) = 18.016 g/mol. Using this molar mass of water, we get,
Example:If we can convert mol to g, then we can do g to g conversion! Given again that
Much the same way as before, but this time, we start with 20.16 g of H2, This time, you need to cancel the gH2 in the denominator. When you see a gram of something, you should always think about molar mass first. The molar mass of H2 is 2 X (1.008 g/mol) = 2.016 g/mol. Then,
Given a balanced chemical equation, you can relate product mass from the reactant mass! Try the following questions!
Example:If 5.0 g of molten magnesium (Mg(l)) is used in the following reaction, how many g of titanium (Ti) is produced?
Example:If 5.0 g of water is produced in the following reaction, how many g of C2H2 did you start with?
Example:If 5.0 g of calcium (Ca) is used in the following reaction, how many g of vanadium (V) is produced?
Calculating the Yield
The calculations we did above for the product mass is called theoretical yield. The product that one actually obtains from experiment is called acutal yield. Usually, actual yield is less than theoretical yield, and we often calculate % yield. where mactual is the actual yield in g, and mtheoretical is the theoretical yield in g. Here is how you might use it.
Example:What is the % yield of H2O if 20.16g of H2 is reacted with O2, and produced 155 g of water, given the following reaction:
We know we started with 20.16 g of H2, and it produced 155 g H2O. It means that 155 g is the actual yield. And then, we need to calculate theoretical yield of H2O using 20.16 g H2. The calculation is done in the previous example: Then, the % yield is,
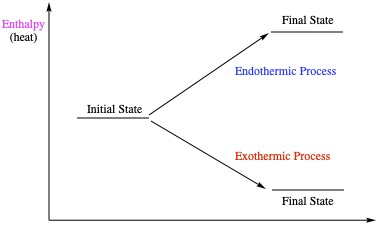
Free Energy and Rxn Rate
Energy = ability to do work.Work = moving an object with a mass by a force to some distance away. Heat is also energy. In chemistry, many reactions deal with heat. Only gas reactions involve with mechanical work.
Heat = Enthalpy = heat energy.
Free Energy = The energy that makes reaction go spontaneously.
For example, a battery is connected to a LED light, it turns light immediately. Ice melts at room temperature. Hydrocarbon is combusted (burned) to produce CO2 and water. These are all spontaneous reactions.
 |
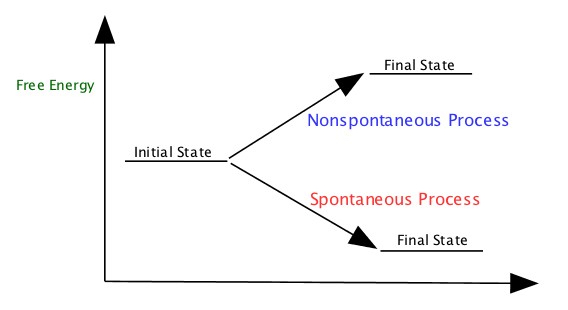 |
| ΔH > 0 is endothermic process ΔH < 0 is exothermic |
ΔG > 0 is nonspontaneous ΔG < 0 is spontaneous |
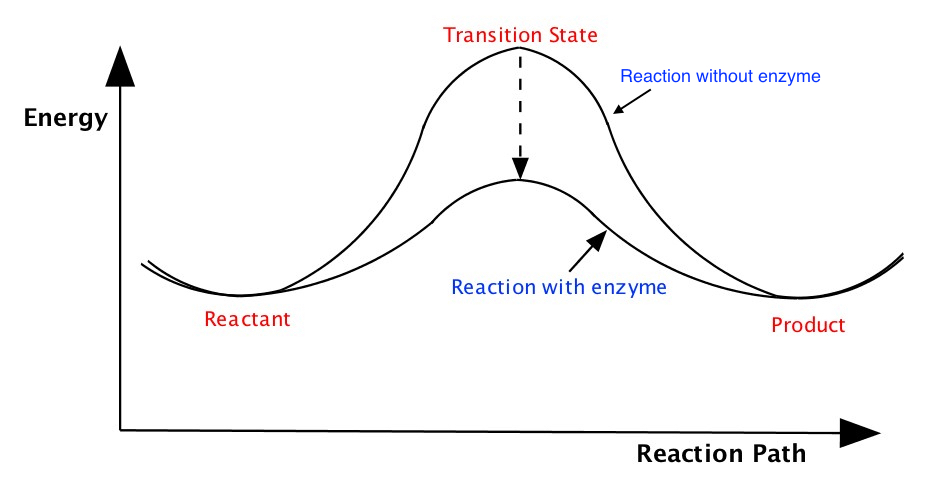
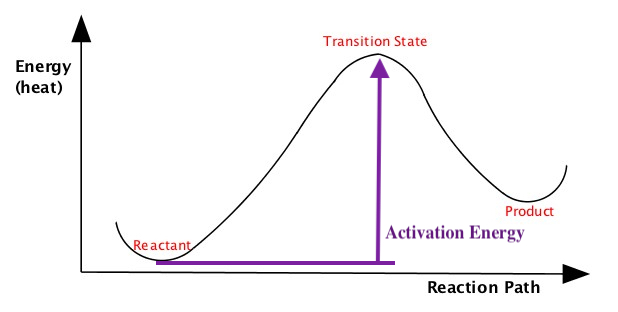
Energy Diagram
We often portray how reactions proceed by illustrating energy in terms of reaction path, similar to the one shown above explaining heat of endothermic and exothermic processes or the free energies of spontaneous and nonspontaneous reactions, but little more detail.The following pictures illustrate energy of a reaction as it proceeds from left (reactant, which is the initial state) to right (product which is the final state). The energies of the reactant and product are at the bottoms of the "U" shapes in the diagram. As you can see, the energy in the middle part is higher than the reactant and product. This is called transition state.
 |
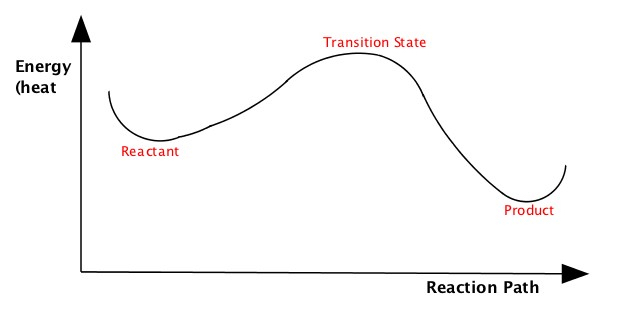 |
| Endothermic Reaction | Exothermic Reaction |
These diagrams tell you is that the reaction proceeds by changing the geometry of the reactant gradually, which makes the enegy goes higher, to become the transition state where the energy of the reaction is highest, then proceed to become the product, where the energy is stable. The energy of the transition state compared to the energy of the reactant is called activation energy is literally the wall that prevents reaction to go quickly. The rate of reaction slows down as baarrier height becomes larger. As I said combustion of hydrocarbon to produce CO2 and water is a spontaneous reaction, but you know just mixing the hydrocarbon with O2 would not combust. This is because of the activation energy of the combustion process is quite high, preventing from sustained combustion. You need a spark of some sort to make the combustion to start. For example, you see the spark on your gas stove or a spark plug in your car to initiate ignition.
Enzyme is a catalyst, made of protein that facillitate reactions. In terms of energy diagram of a given reaction, the enzyme makes the reaction proceeds at much faster rate by reducing the barrier height. The situation is given below: