Gases and Pressure
Gas properties are measured in 4 variables:These specify the characteristics of a gas.
Barometric Pressure
Atmoshere has air pressure. Because of gravitational pull of Earth, air has the highest density at the sea level, hence higher pressure.
Pressure is a force exerted by the molecules of gas to an area. We can measure atmospheric pressure by using a barometer. Torrichelli in mid 1600s made the following set-up by using mercury, Hg, to measure barometric pressure.
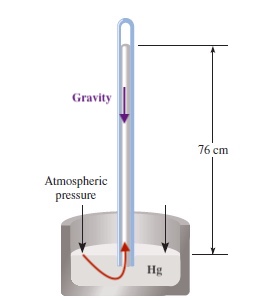
At the sea level, the height of column is quite stable at 76 cm or 760 mm, deviating only few mm day to day. The 1 mm in mercury column is called 1 torr, and when the barometric pressure is 760 mmHg, we define it as 1 atmoshpere (atm) of pressure.
Manometer measures the pressure of gas. The device is a round-bottom flask connected to a bent glass tube such that one can place mercury in to measure the pressure of a gas in comparison to the atmospheric pressure.
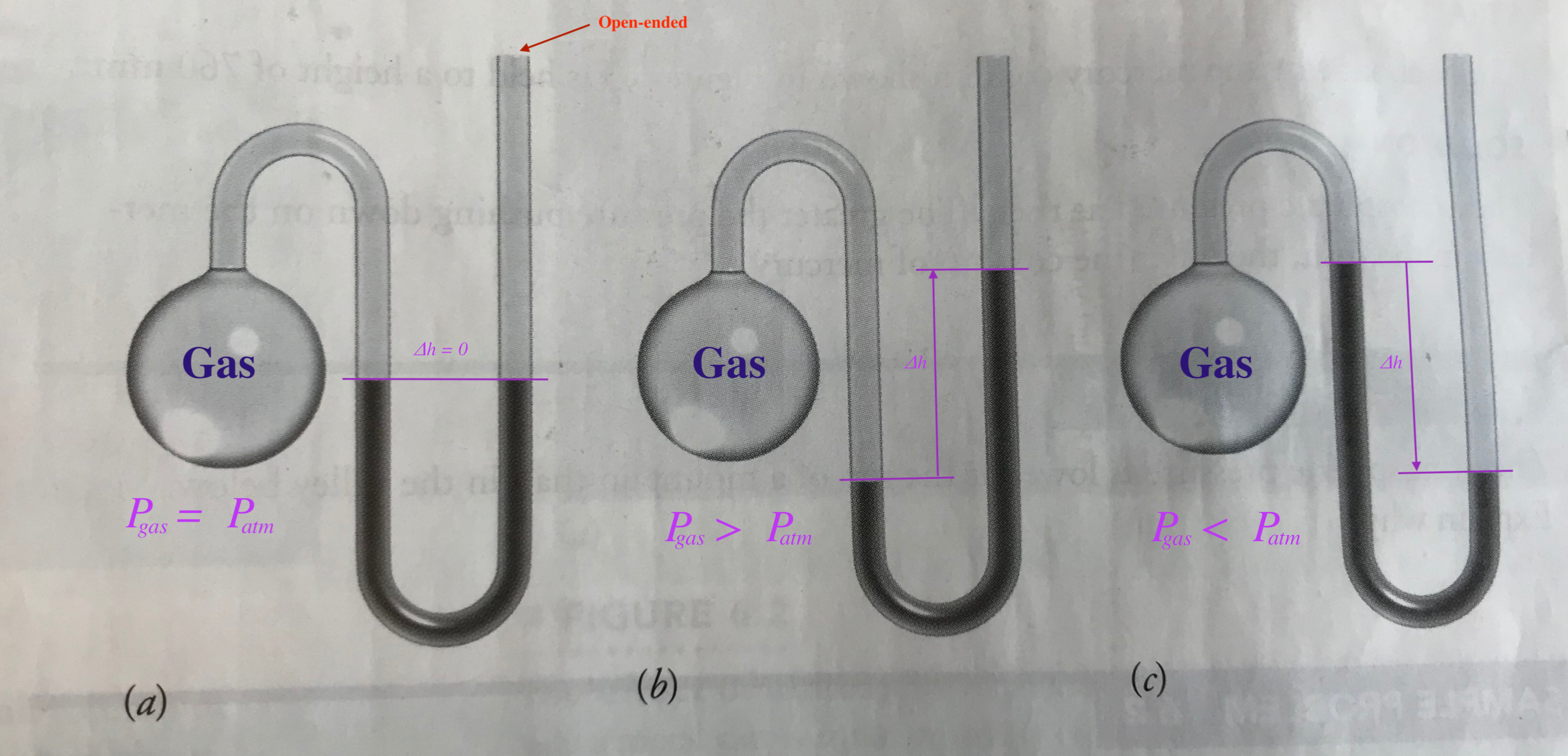
The Gas Laws
The Ideal Gas Law combines 4 different limited laws of gas.
With these laws, we can comnbine to obtain
| PV = nRT |
We can change the condition of gas from its initial state to the final state. We can do this, because R is a constant. Solve for R, you get R = (PV)/(nT). The ratio of PV to nT is always R.
Example:a) What is the volume of 1.00 mole of methane (CH4) measured at 1.00 atm and at 0.00°C? b) What is the volume of 1.00 mol of butane (C4H10) measured at 1.00 atm and at 0.00°C?
a) Since PV = nRT, we rearrange it to solve for V, Before using the above, we need to convert 0°C to Kelvin temperature. T(K) = T(°C) + 273, therefore T = 273K. Then,
b) The answer is the same as above. Since there is no distinction between molecules of gas, if the condition is the same, they all behave (in this case the volume) the same way!
Partial Pressure
Given that volume and temperature are constant, pressure is additive.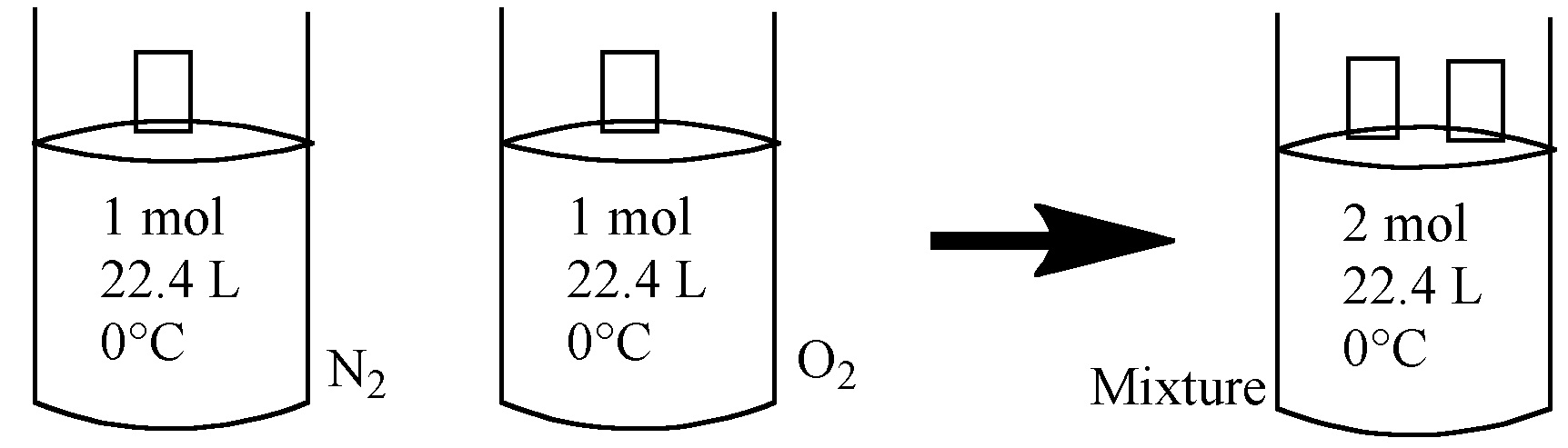
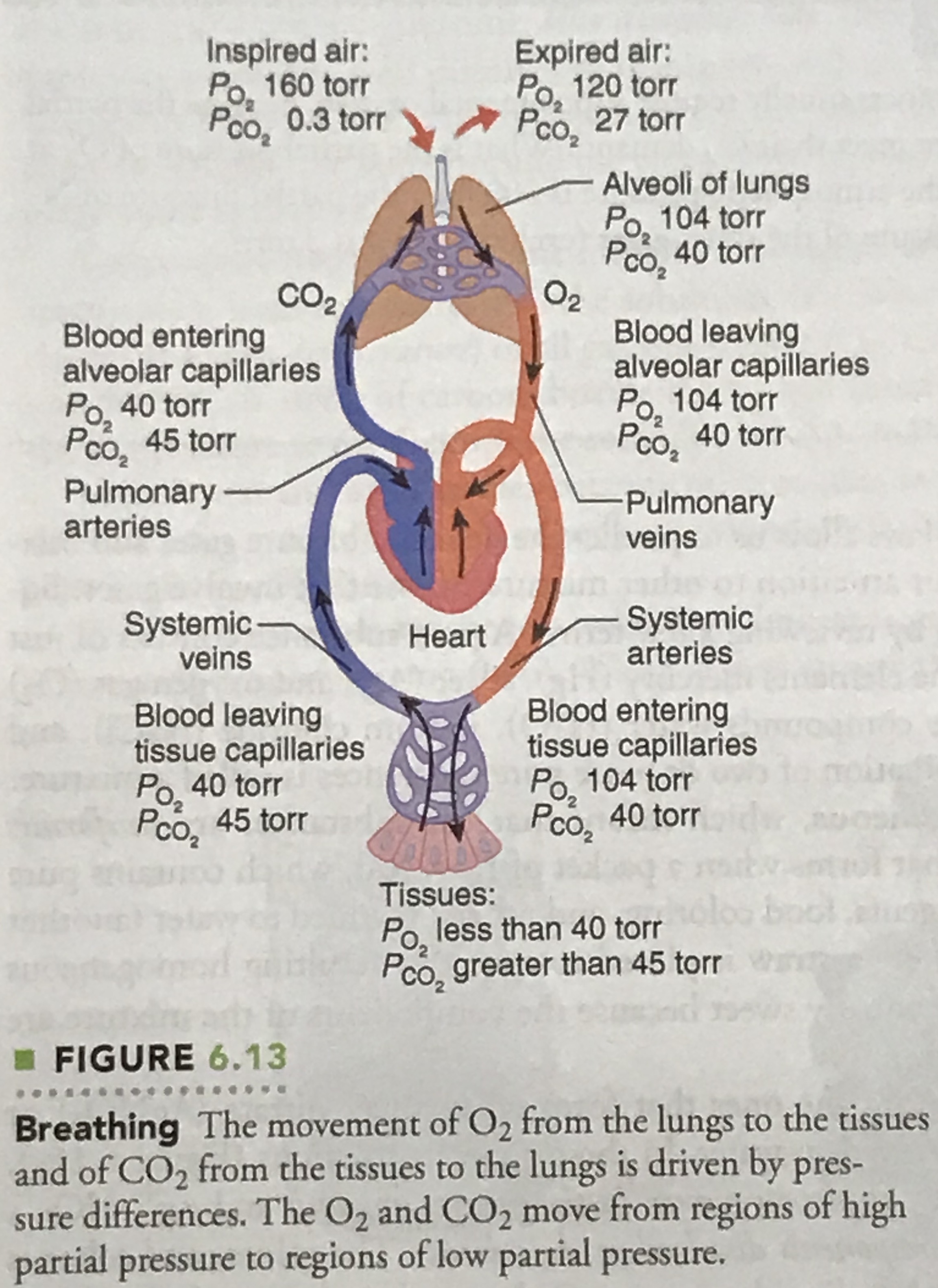
| Location> | Partial Pressure | |
|---|---|---|
| Air | ||
| Alveoli | ||
| Blood leaving alveoli | ||
| At tissue level | ||
| Back to alveoli | ||
| Exhaled air |
Example:A mixture of gases contains 0.75 mol of N2, 0.25 mol of O2, and 0.20 mol of He. What is the partial pressure of each gas, if the mixture is held in a container with 50.0 L at 300 K? What is the total pressure?
First, let's see what we have. We're talking about gases, which means that we are dealing with IGL, PV = nRT. Since Ptotal = Sum of all Pi, we can solve for P = nRT/V.
Solutions
Soution is a two-component system of mixture:
Mixture has two types:
Solubility = how much solute would dissolve in solvent
soluble substance = dissolves in the solvent and makes homogenous mixture.
insoluble substance = doesn't dissolve in the solvent. Two different phases, solid (from solute) and liquid (from solvent).
Then, which substance dissolve in a certain solvent?
| Like dissolves like |
Here is the solubility of alcohols in water. Alcohol has OH group, which can hydrogen-bond to water to dissolve. As the chain length gets longer, it becomes more non-polar (fat-like) thus reducing the solubility.
| Name | Formula | Solubility in water |
|---|---|---|
| Methanol | CH3OH | Completely |
| Ethanol | CH3CH2OH | Completely |
| Propanol | CH3CH2CH2OH | Completely |
| Butaanol | CH3CH2CH2CH2OH | 74g/100g H |
| Pentanol | CH3CH2CH2CH2CH2OH | 27g |
| Hexanol | CH3CH2CH2CH2CH2CH2OH | 6 g |
| Heptanol | CH3CH2CH2CH2CH2CH2CH2OH | 1.7 g |
| Octanol | CH3CH2CH2CH2CH2CH2CH2CH2OH | 0 g |
Precipitation Rxns
If the product of a reaction forms a solid, it is classified as precipitation reaction. We saw in the lab #5 that:
Solubility of Gases in Water
The carbonated drinks, such as coke, is made of sugar water and carbon dioxide dissolved in the solution. You know from experience that when pressure is high more CO2 is dissolved. This is called Henry's Law.
Organic and Biochemical Compounds
Biological processes are carried out in the aqueous environment. Each cell is confined to have efficient biochemical processes. Nature chose to utilize the phase separation between water and hydrocarbon to make the confinement, called cell membrane.
Cell membrane is made up of amphiphilic (or amphipathic) compounds that have property that they dissolve both in polar solvent (water) and non-polar solvent (fat). The polar compounds that dissolves in water is called hydrophilic.
There are several different kinds of membrane lipid. Here one of them is shown.
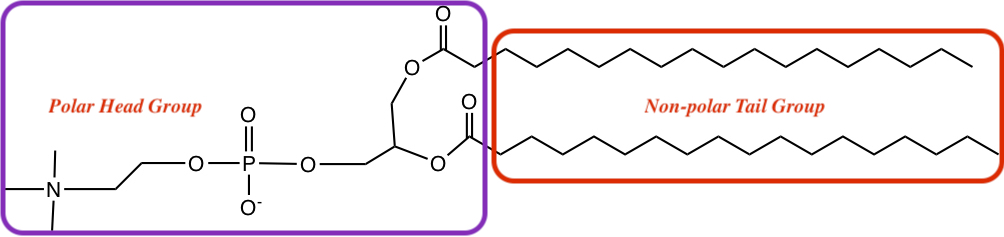
There are few things noticeable:
Concentration
Remember from previous chapters, solution = two-component systemSmall part = solute
Large part = solvent
Concentration in general tells how much solute is in given solvent.
Percent, Parts per Thousand, Parts per Million, and Parts per Billion
You can even think about density to be a kind of concentration. In this sense, weight/volume percent is Instead of multiplying with 100, and multiply with 1000, then it becomes weight/volume parts per thousand (ppt) What if multiply by 1 000 000, it becomes weight/volume parts per million (ppm) You can go even here by multiplying by 1 000 000 000, to weight/volume parts per billion (ppb).
Example:Thalidomide, sold to alleviate morning sickness, is a teratogenic agent that caused severe birth defect in infants in the 50s and 60s. The structure of thalidomide has two forms as shown below.
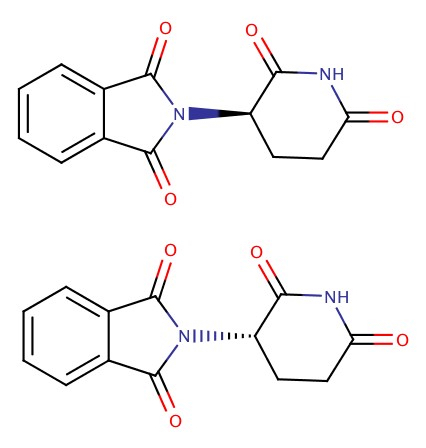
The definition of ppmw/v is as follows. We need first to convert 76 kg weight to volume in mL. It is more convenient to use 985 X 10-6 kg/cm3, since 1 cm3 = 1 mL. Then, Use this result, and if we place 50 mg which is 50 X 10-3 g into the ppm equation. The concentration in ppm is a small quantity. However, effect of the drug is very dramatic.
Molarity
Molarity, M, is one of the most used unit of concentration in chemistry. It is difined as I like using the mol/103mL simply because it is more covenient. By knowing the concentration of solution, one can convert # of moles of solute to volume of solution, and vice versa.
Example:Calculate the molarity of solution if you dissolve 10.0 g of HCl into 200 mL of water.
Since the definition of molarity is So, we need 10.0 g converted to mole (in the numerator) and we need to convert 200 mL to L. and Put them together,
Example:How many moles of HCl are there in 50.0 mL of 1.37M HCl solution?
Simple dimensional analysis yields:
Equivalent
Equivalent = # of moles of charges that one mole of solute contributes to a solution. So, if you have Na+ solution, 1 molNa+ = 1 Eq,1 molMg2+ = 2 EqMg2+
1 molOH- = 1 EqOH-
1 molCO32- = 2 EqCO32-
1 molPO43- = 3 EqPO43-
1 molMg2+ = 2 EqMg2+
Example:The normal serum concentration of chloride ion (Cl-) is 95-107 mmol/L. Convert the range into mEq/L.
Since 1 molCl- = 1 EqCl-, then it also must mean that 1 mmolCl- = 1 mEqCl-. Therefore, 95-107 mmol/l = 95-107 mEq/L.
Example:How many mEq of bicarbonate (HCO3-) are present in a 75.0 mL of blood serum sample with a concentration of 25 mEq/L HCO3-?
You can cast this into dimensional analysis:
Dilution
Making a less concentrated solution from more concentrated solution is called dilution. The higher concentration solution is often called stock solutioin. This is best described by examples:
Example:Let's say that you have 500 mL of 1.00 M solution of HCl. If you add 250 mL of water into the solution, what is the new concentration?
Since M = mol/L, and originally you have 500 mL and you added 250 mL. Thus you have 750 mL = 0.75 L altogether now. So, you have the denominator. Now you need to know how many moles of HCl there are in the original solution. Do dimensional analysis. Therefore,
Example:You are asked to prepare 200 mL of 0.100 M HCl from 1.20 M HCl. How many mL of stock solution do you need?
This is a dimensional analysis problem. So, you take 16.7 mL of the HCl stock solution and place it in a 200 mL volumetric flask, and put water to the 200 mL mark. The volumetric flask is a flask that has only one measurement line.

Colloids and Suspensions
Suspension = relatively large particles (larger than 1 μm) suspended in solution
Colloid = particles are much smaller(1 nm - 1 μm), but the solute is not dissolved.
- Fog is aerosol, which is liquid droplets suspended in gas(air).
- Whipped cream is called foam, which is gas bubbles suspended in liquid.
- Mayonnaise is an emulsion, which is liquid droplets are suspended in liquid.
Solution = solute (less than 1 nm) is fully dissolved
Diffusion and Osmosis
Motion of molecules from relatively more concentrated to lower concentration region is called diffusion.
Osmosis = motion of water from lower solute concentration to higher solute concentration solution.
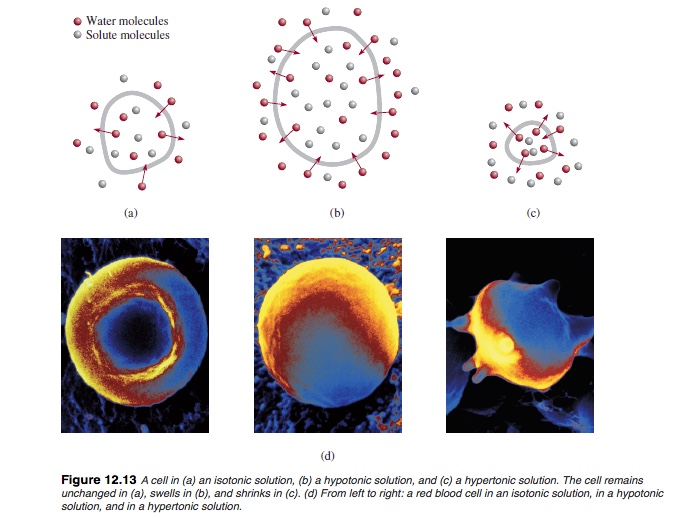
Colligative Properties
Properties of solution that depends only on the concentration regardless of the kinds of solute. There are 4 types:- Osmosis
- Boiling point elevation
- Freezing point depression -- responsible for melting of ice on the streets of NYC in winter (spreading salt on snow covered street).
- Vapor pressure lowering -- this is why we can moon-shine!
Among the colligative properties, osmosis might be most interesting to you. Kidney dialysis is to utilize osmosis to eliminate unwanted and toxic substance that are usually filtered in your kidney. Patients with kidney malfunction has to get rid of the toxins in their blood.
In order to clean the blood, the blood of patient is filtered using semipermeable membrane using the dialysis solution, which is isotonic to blood. Since the concentration of toxin is higher in the blood, the toxin would move to the side of the dialysis solution. Osmosis in action!