Acids and Bases
From our experience:Acid = tastes sour
Base = tastes bitter, and feels soapy.
Brønsted-Lowry Acids and Bases
Acid = donates H+Base = accepts H+
Using this definition, the following reaction, ammonia reacting with water is:
| NH3 | + | H2O | ⇄ | NH4+ | + | OH- |
| Base | Acid | Acid | Base |
In the forward direction, proceeding to right, NH3 accepted H+ to become NH4+, therefore NH3 is a base, while H2O donnated H+ to NH3 and H2O is an acid.
In the reverse direction, the reaction proceeding from right to left, NH4+ is an acid and OH- is a base.
With the similar argument, we can identify the compound to be acid or base in the following reaction.
| HSO4- | + | HPO42- | ⇄ | H2SO4- | + | PO43- |
| Base | Acid | Acid | Base |
Equilibrium
As shown above, equilibrium is a reaction that can go both directions, forward and reverse. And, all compounds in the reaction are present at a given time.Equilibrium Constant
Even though it can go both ways, the concentration of the compounds are given by the so-called equilibrium constant, Keq .
For a given reaction,
For example, acetic acid is in equilibrium with acetate ion such that
| If [reactant] > [products] | Keq = small or Keq < 1 |
| If [reactant] = [products] | Keq = 1 |
| If [reactant] < [products] | Keq = large or Keq > 1 |
Le Châtelier's Principle
Several factors that upset (or affect) equilibrium.
Ionization of Water
Even water can ionize! according to the following.| H2O ⇄ H+ + OH- | Keq = Kw = 1.0 X 10-14 |
The pH Scale
Even though, water is not a very good acid, there are some H+ available in water. In order to keep track of relatively small numbers into much more legible way to express, the biochemist Sørensen at Carlsberg Laboratory (associated with the Danish beer company) came up with the pH scale. The pH scale runs from 0 - 14. The range of pH=0 - 7 is acidic and 7 - 14 is basic. Therefore, in the pH scale, more H+ is available for a given solution as the value of pH become smaller. The opposite is true that as the value of pH increases less H+ is available. The following figure illustrates the relationship between pH and pOH as well as the respective concentrations.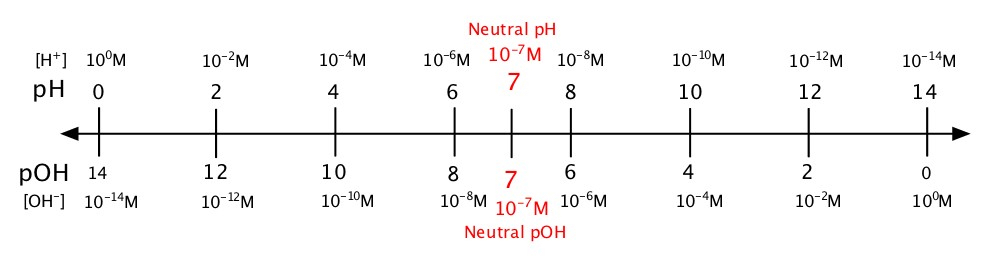
| H2O ⇄ H+ + OH- | Keq = Kw = 1.0 X 10-14 |
Example: What is the pH of a solution if [H+] =1.5 X 10-7M?
Since pH = -log[H+], we have
Example: What is the H+ concentration, [H+], if the solution pH = 5.5?
Inverse of the pH equation is [H+] = 10-pH, therefore,
Since pH + pOH = 14, pH = 14 - pOH, or pOH = 14 - pH, we can handle pOH. From the similar definition, Also, in terms of concentration,
Example: What is the H+ concentration, [H+], if the solution pOH = 5.5?
pH = 14 - pOH = 14 - 5.5 = 8.5. Then,
Acid and Base Strength
Different acid (base) produces different amount of H+ (OH-). Some produces a lot, and some don't. Such determination is done by using acidity constant, which is just another Keq written for acid equilibrium.
For example of HBr(aq) or hydrobromic acid,
| HBr ⇄ H+ + Br- | pKa = -9 | |
| CH3CO2H ⇄ H+ + CH3CO2- | pKa = 4.74 |
By looking at HBr and CH3CO2H examples above, and we know that HBr is a strong acid, indicated by the large value of Ka, than acetic acid, we can conclude that when pKa is small the acid is stronger. Citric acid, shown below, is polyprotic acid, meaning that there are three protons that contribute to acidity.
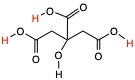
- pKa1 = 3.13
- pKa2 = 4.76
- pKa3 = 6.39
Therefore, citric acid is more acidic than acetic acid. The second proton comes off of the citric acid and the acetic acid is about the same pH.
Example: Consider the initial condition below. These are the weak acid, and its Ka = 1.5. Predict the equilibrium condition.
Neutralizing Acids and Bases
When acid and base react each other, it forms water, as follows.
Titration
The reaction is generally carried out by titration where unknown concentration is determined by the volume of known concentration added.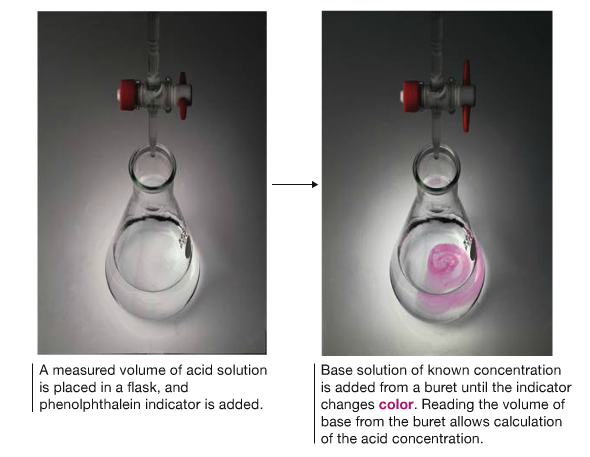
Here are several examples of calculations involving titration.
Example: How many mL of 2.0 M NaOH is required to titrate 20.0 mL of 1.5 M HCl?
The reaction between HCl and NaOH, you can write in two ways. One is
This question can be casted into dimensional analysis. Both concentrations, 2.0 M and 1.5 M, can not be the given, because they each contain mol and L (or mL). So, we start with 20.0 mLHCl, using the first equation.
- First parenthesis -- Molarity of HCl
- Second parenthesis -- Chemical formula of HCl to
break down to H+
- Third parenthesis -- Stoichiometry of balanced
chemical equation
- Fourth parenthesis -- Chemical formula of NaOH
to breakdown to OH-
- Last parenthesis -- Molarity of NaOH
These are things you know, either by inspection or given to you in the question.
If you use the second equation, the dimensional analysis is given below. See the difference? You're calculating the same quantity starting with the same number, thus you should get the same answer! Seemingly, using the first equation looks difficult, but not really. This is because for acid-base reaction is always H+ + OH- → H2O without any deviation! So, I don't have to balance the equation.
Either way of solving the problem is fine.
Example: How many g of NaOH is required to titrate 20.0 mL of 1.5 M HCl?
This time, we are looking for the g quantity of NaOH, and again starting with 20.0 mLHCl. We're still considering the following reaction
Effect of pH on Acid and Conjugate Base [ ]
In weak acid, we know from above that the acid is in equilibrium, such that| HA ⇄ H+ + A- |
Your text said(!) that an interesting relationship exists between pH and the relative concentration of [HA] and [A-], and these are from the mathematics that
| If pH = pKa | [HA] = [A-] |
| If pH < pKa | [HA] > [A-] |
| If pH > pKa | [HA] < [A-] |
Buffers
Buffer is a capacity to resist the change in pH by adding more acid or base when pH = pKa.
pH = pKa happens when the concentrations of the parent acid is the same as those of conjugate base. For example,
[HA] = [A-]
What it means is that if you want to prepare a buffer solution, often the case in biology experiment where you need to keep physiological pH (pH=7-ish) in the solution to carry out.
For example, one of more popular biological buffer is the phophate buffer system where
Example: How would you prepare 100 mL of phosphate buffer system at pH=7.0, if you have a 2.0 X 10-2M KH2PO4 solution and a 2.0 X 10-2M K2HPO4 solution, given that:
First, perhaps you can obtain pKa from Ka so that whether you can actually make a buffer solution or not. This is close to the desired pH. By adding 50 mL each solution, the pH of the solution becomes 7.21. You need to adjust the pH of solution by adding acid, say HCl, while monitoring the pH. Since both solutions have the same concentrations, equal volumes of two solutions should be mixed. Of course, 50.0 mL each solution is mixed.
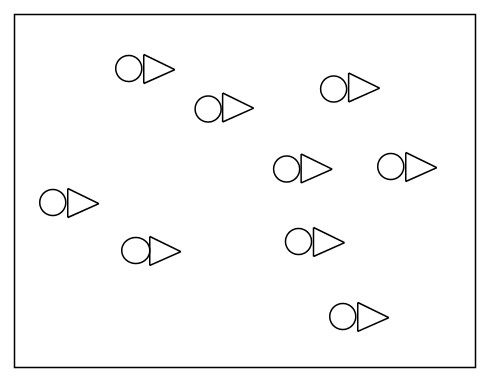
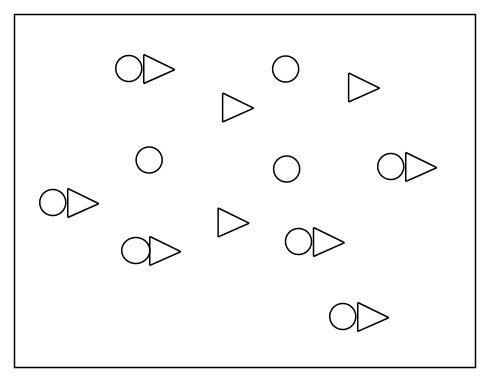
Example: We can make buffer solution pictorially. Let's say that we have an acid in equilibrium as shown below. How many triangle anion is required to form a buffer?