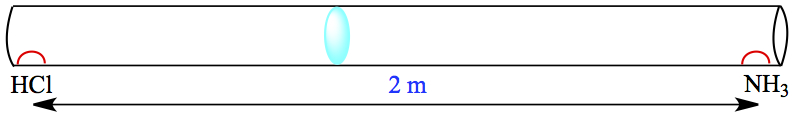
Gas Pressure
Pressure is created by collisions of molecules to the wall
Higher the pressure → more collisions b/w molecules/area/time
Pressure is
with F = force, A = area, m = mass, g = gravity, and ρ = density. In deriving the expression, we used F = ma and m/A = ρh.
Unit of pressure = Pascal (abriviated with Pa) = 1 kgm/s2
Barometric pressure = A column with 1 m x 1 m area and a height extending to the stragosphere containing air exerts 101325 Pa.
Mercury Column Barometer
Torrichelli (1643) used a column of mercury
At sea level height of columen measured = 760 mmHg
Define 760 mmHg = one standard atmosphere or 1 atm
Gravity (g = 9.800665 m/s2)pulls down mercury ⇄ air pressure pushes mercury (ρ = 13.585 g/cm3) up
Example: A barometer is filled with percholorethylene (ρper = 1.62 g/cm3). The liquid height is found to be 6.38 m. What is the barometric pressure in mmHg?
Since PHg = Pper, and expand each P with P = ρgh (make sure to label each with subscripts!)
ρHghHg = ρperhper
hHg = ρperhper/ρHg
hHg = 1.62 g/cm36.38 m/13.595 g/cm3
hHg = 0.760 m = 760 mm = 1.00 atm
Manometer
Manometer = device to measure gas (well, just a flast with bent tube attached as shown)
If the gas inside the flask exerts the same pressure (Pgas) as atmosheric pressure (Patm), the level of liquid in the tube must be leveled. Let's call it, Δh = 0.
As seen on the graph left
If Pgas < Patm → Δh < 0
This is the case for (a)
If Pgas > Patm → Δh > 0
This is the case for (b)
Ideal Gas Law (IGL)
4 gas laws combined. 1) Boyle's Law, 2) Charles' Law, Gay-Lussac's Law and 4) Avogadro's Law
Relationship b/w P and V
Pressure is inversely proportional to Volume
Relationship b/w Volume and Temperature
Volume is directly proportional to Temperature.
When the volume is plotted against temperature in the centigrade scale (a) in the graph, the temperature at which volume becomes 0, is the absolute 0 temperature. So, in (b) we rescale the temperature scale, called absolute temperature scale (the unit is called Kelvin, K).
Pressure is directly proportional to Temperature.
Volume is directly proportional to a number of moles.
| 2H2 | + | O2 | → | 2H2O |
| 2 mol | 1 mol | 2 mol | ||
| ↓ | ↓ | ↓ | ||
| 2 L | 1 L | 2 L |
Combining the four laws
P = cT
V = cT
V = cn
then
PV = ncT, and let us call c as R to obtain
Standard Temperature and Pressure (STP)
T = 0 °C = 273.15 K; P = 760 mmHg = 1 atm
When the volume of one mole of gas is measured, it comes out to be 22.4 L
Molar Volume @STP = 22.4 L.
Initial-Final State Problem
Since R is a constant, the ratio of PV and nT is also constant.
If we have a gas in a container (initial state, labeled with subscript i), we can modify the condition to the gas (final state, labeled with subscript f). These two states are related by R as
Example: We have a vessel containing 2 mol of gas at 100°C and at 1.5 atm,as seen in the picture.
If the pressure of the vessel is reduced to 1.00 atm, what is the temperature of the gas?
Since we didn't change the vessel during the experiment, the volume should be the same, and since we didn't open the vessel, number of gas molecules are the same before and after. It means that
Molar Mass
The molar mass, μ with m and n are mass and # of moles, respecitvely, and its rearrangement is,Substituting above into IGL
Example: A glass vessel weighs 40.1305 g when clean, dry and evacuated. It weighs 138.2410 g when filled with H2O at 25.0°C (ρ = 0.997 g/mL) and weighs 40.2959 g when filled with propylene gas at 740.3 mmHg at 24.0°C. What is the molar mass of propylene?
For propylene gas, we know P and T, so in the above equation for molar mass, we need to obtain m and V.
The mass of the gas is the difference b/w the mass of gass-filled vessel and of the empty vessel, i.e. mgas = mgas+empty - mempty.
The volume of the gas, which equals the volume of the vessel, is obtained by knowing the mass of water in the vessel. The mass of water is converted to volume by its density given.
mH2O = mH2O+empty - mempty
VH2O = mH2O[ 1 mL / 0.997 g ]
Then, substitue these into the expression for molar mass, as
μ = 42.08 g/mol
Gas Density
Rearranging the molar mass equation above, recognizing that m/V is density, ρ, therefore,
@ 0°C ρ = 1.2922 kg/m3
@15°C ρ = 1.2250 kg/m3
@20°C ρ = 1.2041 kg/m3
@30°C ρ = 1.1164 kg/m3
Solve for T in the density equation.
Then, T = (μ P)/(R ρ) = 382.26 K or 109°C
Gases in Chemical Reactions
Relating the amount of gas of reactant or product to other species in the reaction by stoichiometry. Notabean: PV = nRT only applies to gas species in the reaction!!
Gas Mixtures
As long as gases are inert (don't react each other), the total # mol, N, is the sum of the # of moles of each molecular species (n1, n2, ...)
Similarly, nN2 = 0.356909 mol. So, N = n(O2) + n(N2) = 0.446136 mol.
Partial Pressure
The sum of the component gas pressures of a gas mixture, is the total pressure of the mixture.
Ptotal = P1 + P2 + P3 + ...
with Pi is the partial pressure of ith gas.
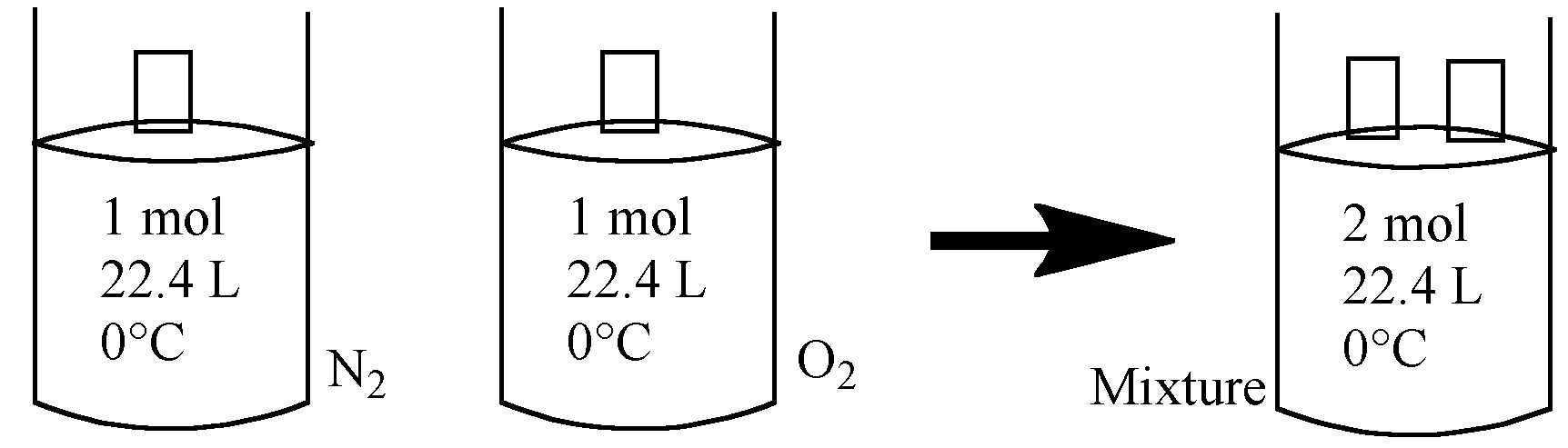
Let's suppose that we have N2 @STP with the volume 22.4 L in one container. It means that we have 1 mol of N2. In antoher container with its volume 22.4 L, we place O2 @STP. So, it also contains 1 mol. Combine these gases, now we have 2 mol of gas, keeping volume V (22.4 L) and temperature T (0°c or 273.15 K) constant. The pressure should then be 2 atm.
So, combined gas has Ptotal = PN2 + PO2
The total pressure is,
Furthermore, we have the relationship
So, if we take the ratio between PN2 to Ptotal you get,
The ratio of mol of ith component and the total # of moles is called mole fraction, χi. Then, we can calculate the partial pressure of N2 by knowing χN2 and Ptotal as
Similar conclusion can be made for VN2 / Vtotal = χN2 if we consider constant P and constant T.
Let's assume at the course of measurement, P, V, and T are constant. Consider Earth as a big container, and its volume doesn't change. Also, if one considers that average pressure at sea level to be fairly constant, we can justify that P, V, and T are constant. Then, we can say that,
It means that χs are
| species | χ | Pi |
|---|---|---|
| χN2 | 0.7808 | 0.768 atm |
| χO2 | 0.2095 | 0.206 atm |
| χAr | 0.0093 | 0.00915 atm |
| χCO2 | 0.00036 | 0.000354 atm |
After reaction, the temperature was returned to 100.0°C, and the mixture of CO2, SO2 and unreacted O2 is found to have a pressure of 2.40 atm. What is the partial pressure of each gas in the product mixture?
Before the reaction was carried out, we have the values of P, V and T, therefore we can calculate the total number of moles of gases in the reactant mixture from PV = nRT. Similarly, we can calculate the total number of moles of gases in the products and excess O2. Let us call nreact and nprod for the respective number of moles.
Each of the gas molecules is stoichiometrically related. Before the reaction, the total number of moles of all gas species is,
We also know the total number of moles of gases after the reaction,
Since 1 mol of CS2 reacts with 3 mol O2, producing 1 mol of CO2 and 2 mol SO2, we can rewrite the nreact and nprod in terms of number of moles of CS2, such that,
Now, in the above two equations, we have two unknowns. If we solve for nO2excess in the second equation, we have,
Substitute the above into the nreact equation, we get,
which is,
Therefore, the number of moles of CS2 is now,
Now that we know the number of moles of CS2, we can relate all other species by stoichiometry in the reaction.
nCO2 = nCS2 = 0.196 mol
nSO2 = 2nCS2 = 0.392 mol
nO2excess = nprod - 3nCS2 = 0.784 mol - 3(0.196 mol) = 0.196 mol
From these, we can finally calculate the product mole fractions, χ.
χSO2 = 0.392 mol / 0.784 mol = 0.500
χO2excess = 0.196 mol / 0.784 mol = 0.250
PSO2 = 2.40 atm (0.500) = 1.200 atm
PO2excess = 2.40 atm (0.250) = 0.600 atm
Kinetic Theory of Gases
IGL at molecular level -- meaning of kinetic energy (thermal energy)
Our model
Using this model we can derive the expression for pressure,
where N is the # of molecules, m is the mass of molecules <ν> is the average velocity of gas, and V, is the volume of the container.
If you want to see the derivation of the pressure expression, click below!
Meaning of Kinetic Energy
From Newton's law of motion, we know that kinetic energy, EK = 0.5 mv2, we can derive expression for kinetic energy for gas, with R = 8.314 J/molK (another way to express IG Constant, also 1 J = 1 kgm2/sIf T → 0, EK → 0
Furthermore, the average speed of the gas molecules can be obtained by rearranging the kinetic energy equation,
Therefore, the speed of molecule depends on the molar mass and temperature. Heavier the molecules, slower the speed at given temperature. The speed is higher at higher temperature.
Effusion Rate
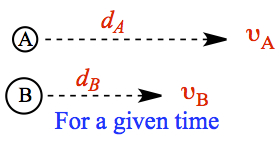
Consider two molecules, lighter A and heavier B, moving in one direction, as shown on left. For a given time interval, distances covered by the molecules differ according to the velocity equation, which depends on molar mass. Lighter molecule travels further for a give time interval, if the two gases are at the same temperature. We can obtain the relationship between the distance traveled is given by the following ratio.