Intermolecular Forces
Dipole Moment
Remember that Fluorine is has the highest value of electronegativity, χ? Due to electronegativity difference, Δχ, b/w two atoms, bond would polarize.
If the Δχ = 0, then the bond is said to be non-polar.
If the Δχ is non-zero, the bond is polar.
So for molecule N=O, we have
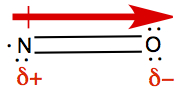 As it is seen, arrow indicates the dipole moment of the bonds between
N and O, and the arrow points toward more negative side, indicated with
δ-, and the other end is more positive side,
δ+. This is so because oxygen is closer to
F in the periodic table than nitrogen.
As it is seen, arrow indicates the dipole moment of the bonds between
N and O, and the arrow points toward more negative side, indicated with
δ-, and the other end is more positive side,
δ+. This is so because oxygen is closer to
F in the periodic table than nitrogen.
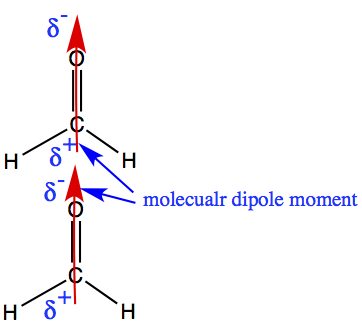
 Adding all Polarities of bonds →
molecular dipole moment, μ
Adding all Polarities of bonds →
molecular dipole moment, μ
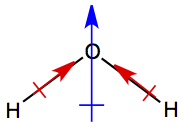
The bond dipole moments of O-H bonds (red arrow) in water points toward oxygen atom. When these two bond dipoles are added together, molecular dipole moment (blue arrow) is produced. As in the bond dipole, the direction the arrow is pointing has partially negative charge, and the opposite end has partially positive charge.
In order to deduce dipole moments of molecule is to know the 3-D structure of the molecule. Then, draw in the bond dipoles (red arrows). We consider C-H and C-C bonds to be non-polar bonds, therefore we don't draw in the arrow for these bonds. After bond dipoles are drawn in, all bond dipole vectors are added, and the resulting vector is the molecular dipole moment (blue arrow ).
For CF4, two of the red bond dipoles form a resultant green dipole in the plane of the computer monitor. For other two bond dipoles, with orange color, form another green resultant pointing in the opposite direction. And, they cancel each other, therefore the moleuclar dipole moment of CF4 is zero.
Intermolecular Forces
Forces b/w molecules
Forces responsible for condensing gas into liquid and liquid into solid
Forces responsible for making different compounds to have different melting point and boiling point.
Four types:
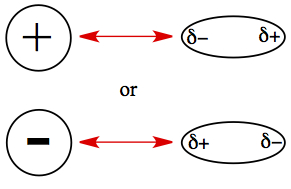 Force exits between ion and molecule with permanent dipole moment. Cation is attracted to the partially negative side of the polar molecule, and anion is attracted to the partially positive end of the polar molecule.
Force exits between ion and molecule with permanent dipole moment. Cation is attracted to the partially negative side of the polar molecule, and anion is attracted to the partially positive end of the polar molecule.
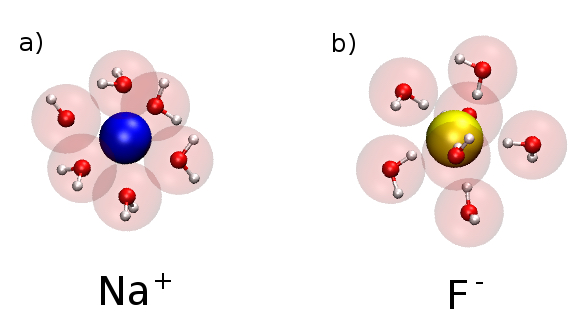 Water surrounds Na+ ion in a) and F- ion surrounded by water in b). The red sphere in water is the oxygen atom. Notice that the oxygen is pointing toward Na ion, and the hydrogen atoms point toward F ion.
Water surrounds Na+ ion in a) and F- ion surrounded by water in b). The red sphere in water is the oxygen atom. Notice that the oxygen is pointing toward Na ion, and the hydrogen atoms point toward F ion.
 Interaction b/w two molecules with permanent dipole moments. The partially positive charge side is attracted toward partially negative side, and vise versa.
Interaction b/w two molecules with permanent dipole moments. The partially positive charge side is attracted toward partially negative side, and vise versa.
So, if you have a bunch of N=O molecules, you'd see the packing of molecules as shown on the left diagram in the figure below. On the right diagram negative-negative interactions and positive-positive interactions, both represented by green arrows, are the repulsive interaction. So the structure is unstable. It'd eventually become the same packing as on the left.
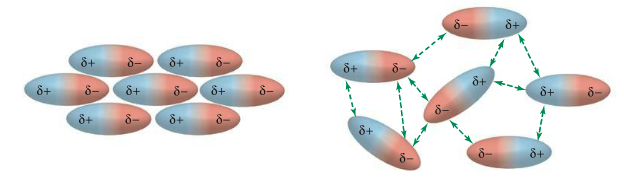
Dipolar vs non-dipolar interactions
| N2 | NO | O2 | |
| Dipole(debye) | 0 | 0.153 | 0 |
| Mass (g/mol) | 28 | 30 | 32 |
| Boiling pt(K) | 77 | 121 | 90 |
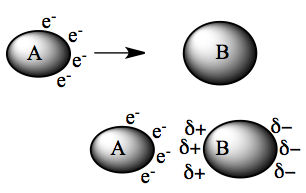
As they are close to each other, B sees the electrons on A, and the electrons on the left-side of B starts to avoid the approaching A (lower diagram on left). This induces polarity on molecule B, which in turn can polarize A as well. This is the source for the attractive force between non-polar non-ionic molecules.
If the molecule is easy to deform → said to be Polarizable.
| Halogen | mp (K) | bp (K) |
|---|---|---|
| F2 | 53.5 | 85.0 |
| Cl2 | 171.6 | 239.1 |
| Br2 | 265.9 | 331.9 |
| I2 | 386.8 | 457.5 |
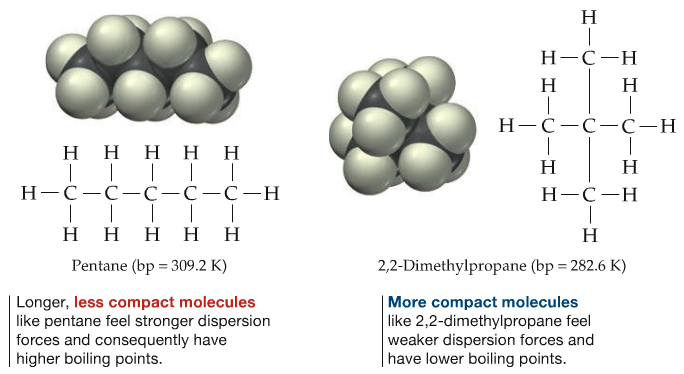 I
I
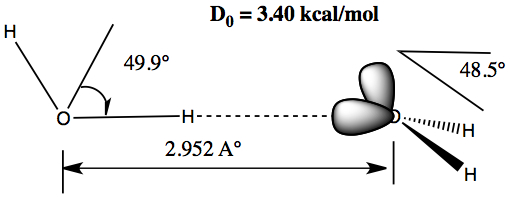
Sharing of hydrogen between two molecules
Each molecule must act as both the donor and acceptor.
The picture on the right is a dimer of water. The molecule on left is the H-donor and the one on right is the acceptor.
The shared hydrogen is co-linear with the lone pair-H-donor.
The binding energy for water dimer is quite small (3.4 kcal/mol). But, this is the force holding two strands of DNA.
Comparison of the strength of intermolecular forces
| Force | Strength in kJ/mol | Strength in kcal/mol |
|---|---|---|
| Ion-Dipole | 10 - 50 kJ/mol | 2 - 12 kcal/mol |
| Dipole-Dipole | 3 - 4 kJ/mol | 0.5 - 1 kcal/mol |
| Dispersion | 1 - 10 kJ/mol | 0.2 - 2 kcal/mol |
| H-bond | 10 - 40 kJ/mol | 2 - 10 kcal/mol |
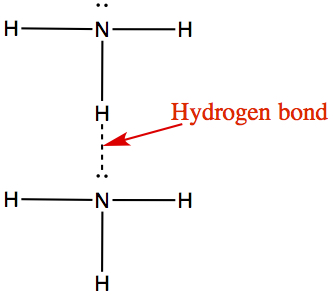
1) Liquid ammonia, NH3, has lone pair electrons and H, therefore it can H-bond to each other. The two molecules can interact in the following way.
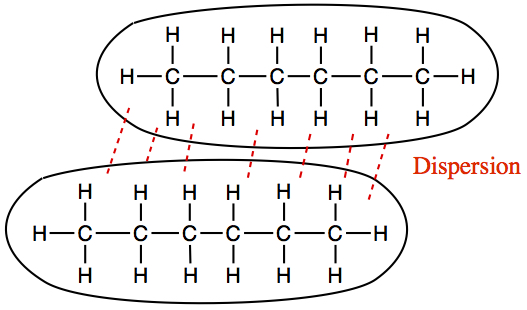
2) C6H14 (hexane) is non-polar and elongated molecule. So, it only has dispersion interaction. In order to liquify, two molecules must be aligned sideways as shown below.
3) Formaldehyde is a dipolar molecule. The dipole-dipole interaction entails lining up the two dipole moments as follows:
Liquid Properties
Surface Tension Energy needed to increase surface area (Cohesive energy)
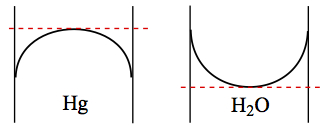 The red dashed line is the meniscus you read for measurements, like measuring the volume of water with graduated cylinder.
The red dashed line is the meniscus you read for measurements, like measuring the volume of water with graduated cylinder.
| Mercury side = Convex | H2O side = Concave |
| Hg Intxn with glass is weak | H2O Intxn with glass is strong |
| Cohesive Energy of Hg is stronger than intxn b/w Hg and glass | Cohesive Energy is weaker than intxn b/w H2O and glass |
Structure of Water
Water is peculiar. Unlike most other liquid, its density in water is higher than the solid state (ice). You know ice floats on top of the water tells you exactly the fact. The reason for high density in water is in the structure of water. The image shown below is the structure of solid water, and they are completely hydrogen-bonded to each other.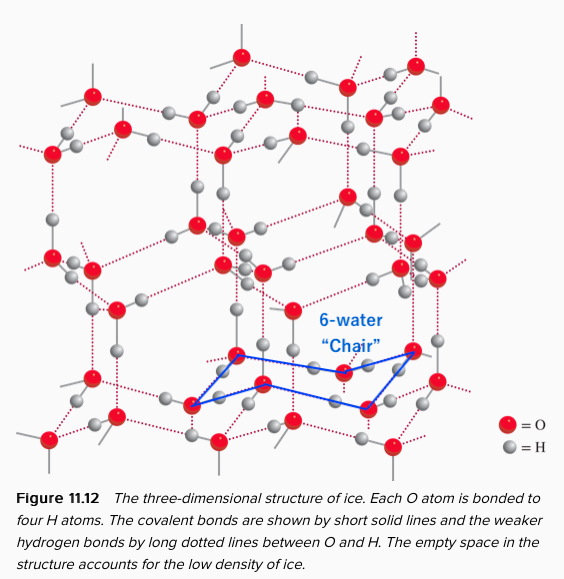
Crystal Structure
Two structural types of solids
Types of Crystaline Solids
| Types | Intermolecular Forces | Properties | Examples |
|---|---|---|---|
| Ionic | Ion-ion force | Brittle, hard, high-melting | NaCl, KBr, MgCl2 |
| Molecular | Dispersion, dipole-dipole, H-bond | Soft, low-melting, non-conducting | H2O, Br2, CO2, CH4 |
| Covalent network | Covalent bonds | Hard, high-melting | diamond, SiO2 |
| Metallic | Metallic bond | Variable hardness and melting points, conducting | Na, Zn, Cu, Fe |
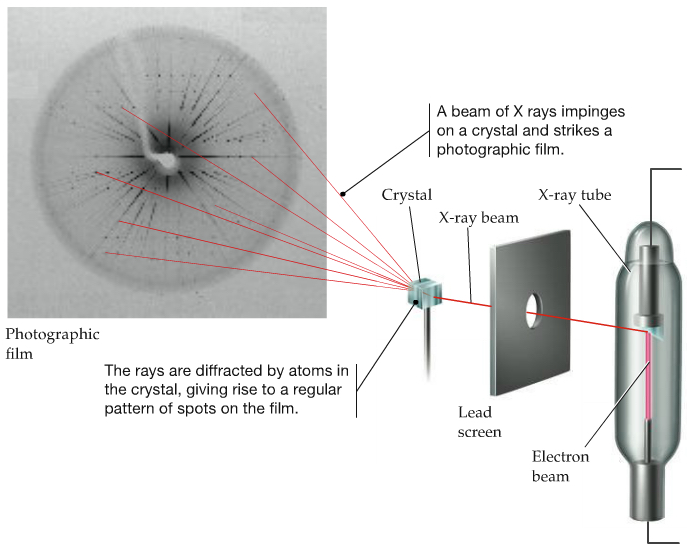
X-Ray Diffraction
X-ray is used to determine the structure of crystal -- very important tool in Biochemistry -- Protein structures are determined this way. The structure of DNA was discovered by this technique.
 |
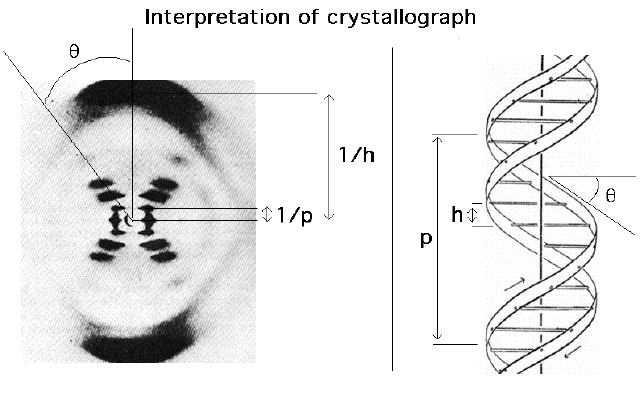 |
| Above diagram shows how the experiment is carried out. X-ray is irradiated onto a crystal. Then the diffraction pattern is photographed. | This is the diffraction pattern of one form of DNA. As can be seen, the structural parameters are obtained from the diffraction pattern. |
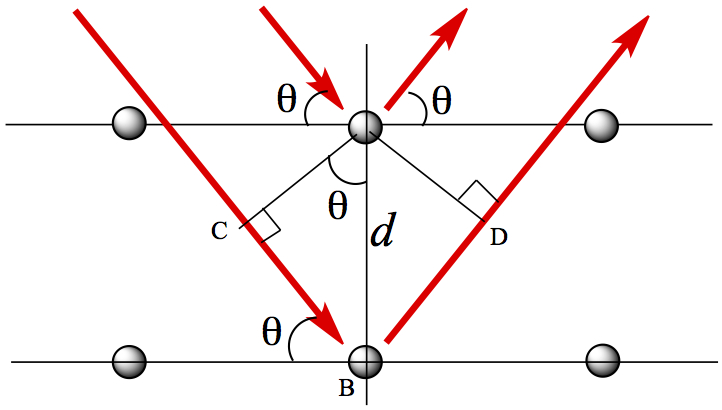
The red arrows from left to fall on atoms indicates incident X-ray, and the red arrows going away from atoms are the diffracted (outgoing) X-ray. The incident rays with the wave length, λ, on the 2nd layer must travel extra distances covered by the lengths, C-B and B-D. The diffraction pattern only appears when the extra distances, C-B and B-D are integral multiples of λ
By using trigonometry, one can show the extra distnaces traveled is:
Then, according to Bragg, the distance C-B and B-D is related to integral multiple of λ, thus one obtians the relationship between incident X-ray wave length and the structural parameter, d is given as
This is called Bragg equation.
Rearranging Bragg equation, we have Here, we have the longest wave length, therefore n = 1. The layer distance, is 282 pm, which is 2.82 Å, thus the wave length is
Crystal Types
Crystalline solids have repeating unit → Unit Cell.
The repeating pattern of unit cells determine the structure of crystal.
Crystal packing
Crystal packing shows how the atoms are arranged in a crystal. There are four types:
| Simple Cubic | 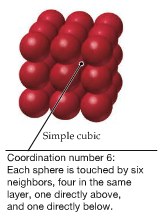 | |
| Body-Centered Cubic | 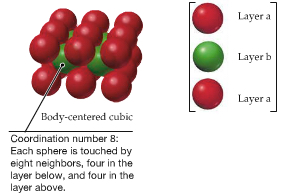 | |
| Hexagonal Closest-Packing | 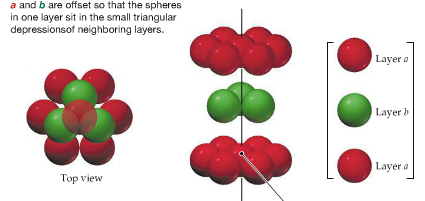 | |
| Cubic Closest-Packing | 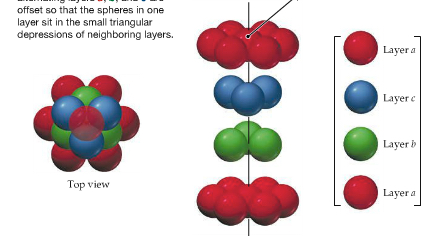 |
Unit Cells
Within the packing pattern, one can consider a unit of repeating cells, called unit cell, which contains fewest possible atoms. For example, simple cubic packing pattern can be considered to have a unit cell with the edge length a and contains 1/8 of atoms at each corner, which means there are 1 atom in the unit cell, as shown below. There are two other types of unit cell within the so-called simple cubic unit cell.3 types:
| Primitive Cubic | 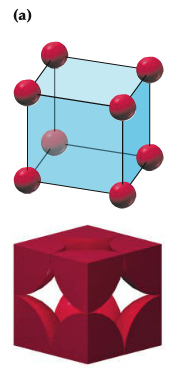 |
| Body-centered Cubic | 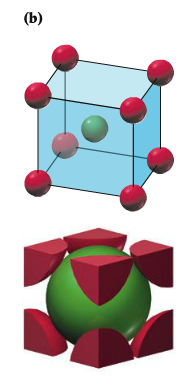 |
| Face-centered Cubic | 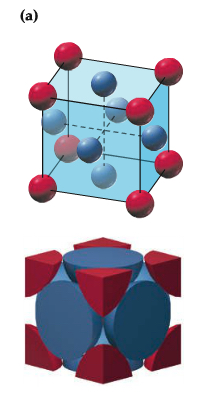 |
In the following diagram, the relationship between the edge length and atomic radius of monoatomic solid that packs in a simple cubic cells.
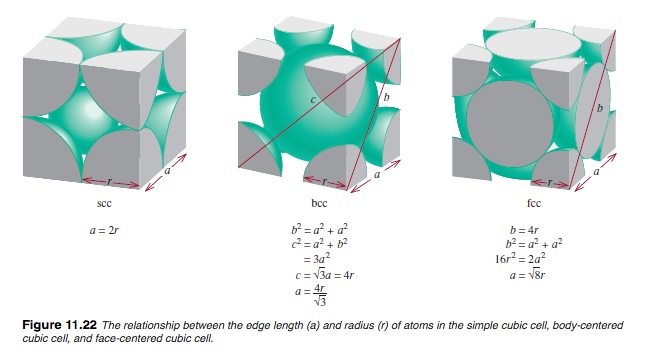
Using the above diagram, we can get the edge length, a, from the atomic radius, r, by using the relationship shown in the diagram above, and it is Therefore, a = 4(1.39 Å)/√3 = 3.210 Å. So the volume of the unit cell is (3.210 Å)3.
We need the mass of W atoms in the unit cell. We can obtain it by counting the number of atoms in the cell and convert them into g, using Avogadro's number.
There are two W atoms in the cell. Then, The volume is (3.210 Å)3, but it needs to be converted to cm3. The density, then is, The measured density is 19.25 g/cm3.
Avogadro's number is relating the atomic mass unit (amu) to gram unit. It means that so many amu would make up to be one gram. Remember that in Chapter 3, we saw that And, we said that the reciprocal of the gram value was the Avogadro's number. It means then the value Therefore, we seek this ratio. First, we obtain amu/cm3, then we obtain g/cm3. Because we can calculate these.
Since we already know the density, which is 3.50 g/cm3, we just need to convert the unit cell dimension to amu per cm3. Then, take a ratio between them to obtain the desired amu to g ratio.
Since there are two barium atoms in the cell, the mass is 2×137.33 amu in the unit cell which makes the density to be Avogadro's number is given by the ratio of atomic mass unit (amu) to the gram quantity (amu/g) according to Chapter 3. The ratio of densities (amu/cm3 to g/cm3) would give us the desired value of amu/g. or Not too bad of agreement.
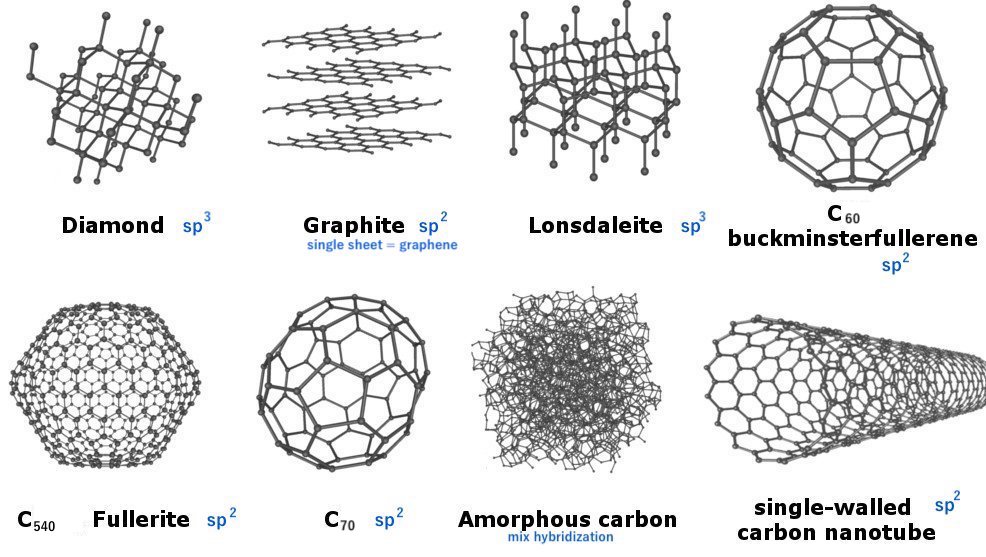
Allotropes = same element with different structural forms
Carbon has 40 allotropes! Graphite (graphene → Nobel Prize for synthesizing), Buckminsterfullerene or bucky ball (Nobel for determining structure), Bucky tubes, diamond, etc.
Some of the allotropes of cabon are shown here:
Phase Changes
Phase Change
A few names associated with phase change:| Fusion (melting) | solid → liquid | Δ H >0 |
|---|---|---|
| Freezing | liquid → solid | Δ H < 0 |
| Vaporization | liquid → gas | Δ H > 0 |
| Condensation | gas → liquid | Δ H < 0 |
| Sublimation | solid → gas | Δ H > 0 |
| Deposition | gas → solid | Δ H < 0 |
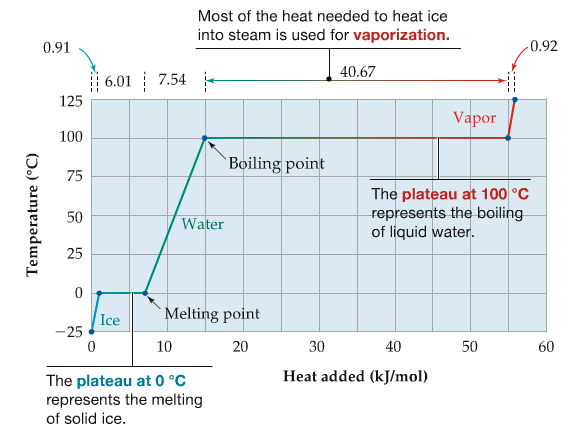
This is a plot of heating curve of water. Plotted is the temperature of water by adding more heat (energy) to the water. Notice there are two plateaus at melting point and at boiling point.
In order for you to make 100° liquid H2O to 100° gas H2O (steam), it needs about 40 kJ/mol of energy.
This is the heat of vaporization, ΔHvap = 40.67 kJ/mol.
Similarly, you see less than 10 kJ/mol is needed to melt ice into liquid → heat of fusion, ΔHfus = 6.01 kJ/mol
The total heat required is given by contributions from bringin ice to 0°C, melting ice, heating liquid to 100°C, converting water to steam, and bringing steam to 120°C, which can be shown in the following: The contributions for qice, qwater, and qsteam, needs , while the contributions from qfus and qvap are to compute q from the heats of fusion and vaporization.
Liquid Vapor Equilibrium
Vapor Pressure ← Dyanmic equilibrium b/w liquid and gas
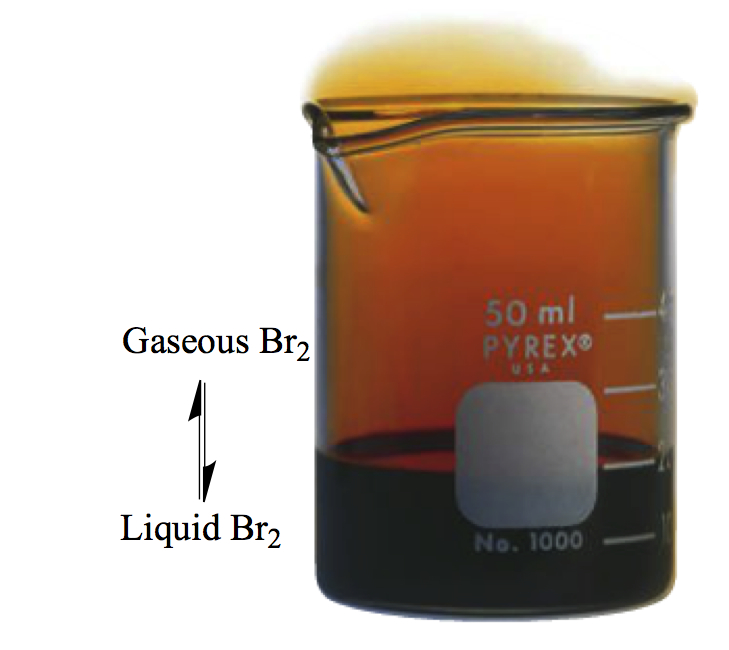
Over the surface of liuquid bromine, you see vapor of gaseous bromine. Liquid and gas establish a so-called dynamic equilibrium.
Ratein = Rateout
Higher the temperature → Higher the vapor pressure
Higher T → Larger EK → Easier to overcome intermolecular intxns.
T > 0 K, there is always vapor exists over solid or liquid.
Clausius-Clapeyron Equation
If you plot Pvap as a fxn of T, you get a picture on the left
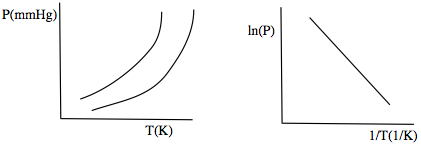
But if you plot ln(P) vs. 1/T, instead, the curve becomes linear!
Linear equation is easier to work with!!!
The relationship b/w ln(P) and 1/T is,
In this equation, R = 8.314 J/molK is used. It means that for T, we should be using Kelvin unit. For pressure, you can use whatever the unit as long as P1 and P2 have the same unit. Δ Hvap is the heat of vaporization (for water, it is 40.67 kJ/mol as we discussed above).
We know water boils at 100°C at sea level at which barometric pressure is 1.00 atm. Let's assign P1 = 1.00 atm and T1 = 100°. So, we are solving for T2. Then,
Substitute #'s in.
T2 = 368.15K, or 95.0°C.
We can go the other way around:
Again, we know water boils at 100°C at sea level and at 1.00 atm barometric pressure. We can make T1 to be the 100°C and P1 to be 1.00 atm. Then, T2 to be 95.0°C and we solve for P2. Exponentiate both sides of the equation, we get
Phase Diagram
Phase diagram shows phases of substance as a function of temperature and pressure.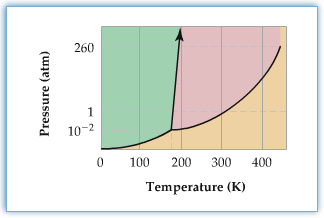
Each solid lines represent phase boundary. For example, the line in the diagram with an arrow is a phase boudnary b/w solid and liquid.
We know:
So, the left-most (green region) is solid, middle part (pink region) is liquid, and bottom part (yellow region) is gas.
Important points and processes on phase diagram
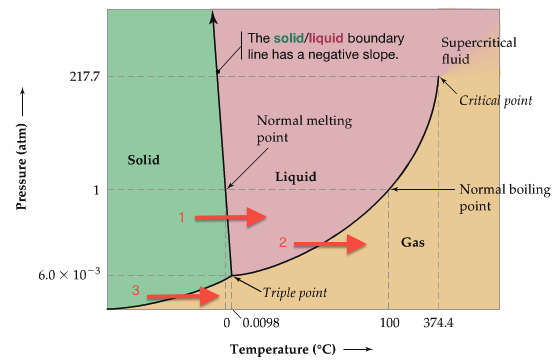
Few important Points on the Phase Diagrams:
Supercritical Fluid is used to decaffinate coffee beans.
Important Processes: