Solutions
Solution is a two-component system.
Energy Changes and the Solution Process
When some solute spontaneously dissolves into solvent, energetics must become favorable. Such energy is called Gibb's free energy, given by the following.
ΔG = ΔH - TΔS
governs the dissolution process. So there are two components:
Enthalpy (Δ H part)
Entropy (Δ S part)
In all cases, Entropy term is favorable for making a solution. This is because a solution is more disorderly than the separate solute and solvent.
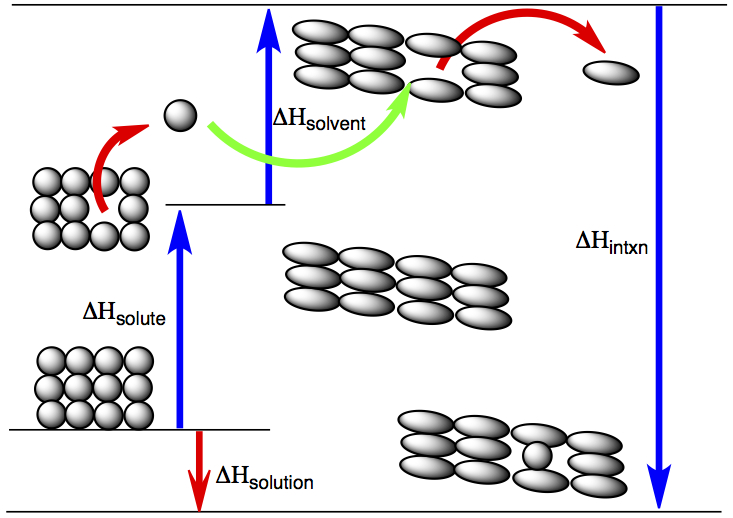
Exo- or endothermicity of the process of dissolving (enthalpy of solution) is explained by the following diagram.
Here is the solubility of alcohols in water. Alcohol has OH group, which can hydrogen-bond to water to dissolve. As the chain length gets longer, it becomes more non-polar (fat-like) thus reducing the solubility.
| Name | Formula | Solubility in water |
|---|---|---|
| Methanol | CH3OH | Completely |
| Ethanol | CH3CH2OH | Completely |
| Propanol | CH3CH2CH2OH | Completely |
| Butaanol | CH3CH2CH2CH2OH | 74g/100g H |
| Pentanol | CH3CH2CH2CH2CH2OH | 27g |
| Hexanol | CH3CH2CH2CH2CH2CH2OH | 6 g |
| Heptanol | CH3CH2CH2CH2CH2CH2CH2OH | 1.7 g |
| Octanol | CH3CH2CH2CH2CH2CH2CH2CH2OH | 0 g |
Units of Concentration
We know already few units of concentrationMolarity (M)
M = molsolute / 1 Lsol'nMole Fraction (Χ)
Χ = molsolute / moltotalMass Percent (%mass)
%mass = gsolute / gtotal x 100%Molality (m)
m = molsolute / 1 kgsolvent
Density (d) can also be considered as concentration unit, in this respect.
Conversion between these units is accomplished, none other than, yes, Dimensional Analysis!
Example: Consider an aqueous solution of CO(NH2)2 with its d = 1.00 g/mL. Given that m = 2.577 m, what are the %mass of solute, ppm of solute, mole fraction of solvent, and molarity?
μCO(NH2)2 = 60.062 g/mol
Therefore, total mass, mtotal= 1000 g + 154.779774 g = 1154.779774 g, then
For ppm, instead of multiplying 100 to the ratio, 106 is mulitiplied to obtain
1.340 x 105 ppm.
For χ, you need the total number of moles, which is the sum of molsolute + molsolvent. The # of moles of solvent is,
Combining the two contributions, moltotal = 55.560 + 2.577 = 58.10832 mol, so χsolvent is given by χ = 55.560 mol / 58.10832 mol = 0.9556.
For molarity, assuming the solution density is 1.00 g/mL, we can convert total mass of solution, which was calculated to be 1154.8 g = 1.1548 L in volume, thus to obtain M = 2.323 M.
Example: The density of an aqueous solution containing 10.0 % ethanol (C2H5OH) by mass, is 0.984 g/mL. Calculate the molality, molarity, and volume of the solution would contain 0.200 mole of ethanol?
There are 10.0g ethanol and 90.0g water in the solution. We should convert the mass of ethanol into moles. We need also to convert the denominator for molality, which is 90.0 g to kg, that is 0.0900 kg. Therefore, For molarity, the solution volume is obtained from 100 g of the solution. Therefore, The last bit is asking for volume, thus we use molarity.
Factors affecting Solubility
Effect of Temperature
Generally, higher the temperature more solute can be dissolved.
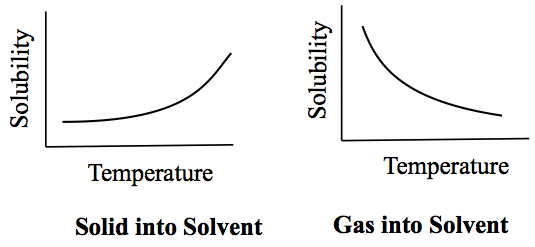
Solid dissolving in water generally increases, while gas in water decreases.
Except:
NaCl doesn't really change its solubility over the range of 0°C to 100°C. In addition,
Ce2(SO4)3 actually decrease its solubility in the range of 0°C to about 40°C.
Effect of Pressure
The concentration of gas is proportional to the gas pressure applied.
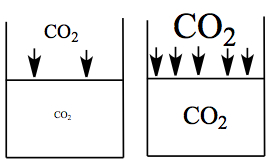
Therefore, we have
where [ ] is the concentration of gas in solution, and k is called Henry's law constant with M/atm unit.
The constants differ for different gas molecules. For example, the Henry's law constant for O2 is 1.3 X 10-3 mol/Latm, while for N2 is 6.8 X 10-4 mol/Latm.
Example: Assuming that the partial pressure of oxygen and nitrogen in air to be 0.20 atm and 0.80 atm, respectively, calculate the mole fractions of oxygen and nitrogen in water at 298 K.
We should first know the concentration of the O2 and N2
that are dissolved in water by using the Henry's law.
Similarly for N2, c = 5.44 X 10-4 M.
Assuming we have 1 L of solution, we have 2.6 X 10-4
molO2 and
5.44 X 10-4 molN2 in the solution.
So, the solution is a three-component system now. It means that in order for
us to calculate the mole fractions, we also nee to know the number of moles
of the solvent. Assuming that the density of water is 1.00 g/mL, we have
Therefore,
The total number of moles is 55.5068
Colligative Properties
Physical changes induced by small amount of solute. They depend only on the concentration, and not the nature and/or type of solute.4 types
Raoult's Law: Vapor Pressure Lowering
In comparison to pure liquid, solution has lower vapor pressure. The vapor pressure depends on the mole fraction of solvent, χ1.
For solvent with non-volatile solute
where subscripts 1 indicates that it is for solvent, and ° (the circle on P) indicates for the vapor pressure of pure solvent.
You can put the above equation in terms of change in pressure by introducing the solute into pure solvent in a following manner. Since there are only two component, χ is of two parts χ1 for the solvent and χ2 for the solute. Furthermore, χ1 + χ2 = 1. So, χ1 = 1 - χ2, and substitute this into P1 = P°1 χ1 yields Therefore,
Example: How many grams of glucose, C6O6H12 (μ=180.2 g/mol), is required to obtain the vapor pressure change of 1.4 mmHg in 460 mL of water at 30°C? The vapor pressure of pure water at 30°C is 31.82 mmHg. Assume density of water at this temperature is 1.00g/mL.
It means that we know ΔP to be 1.4 mmHg. Then, using the last equation, we can arrive at the mole fraction of glucose (χ2).
Substituting the numbers in, yields: Since χ is a mole fraction, and we know the amount of water we can obtain the number of moles of glucose in the following way: So, we need to know the number of moles of water, for which we have 460 mL. Substitute this into the mole of glucose is, then:
For solvent with volatile solute
The total pressure now contains, not only the solvent vapor, but also contains solute vapor. Therefore, the total pressure is a sum of the two contributions
Boiling Point Elevation
Boiling point is higher in solution than that of pure solvent.where ΔT is the change in boiling point, m is the molality of sol'n, and kb is the boiling point elevation constant. For water as solvent, kb is 0.516 °C kg/mol.
For ionic solute, the equation must be corrected by van't Hoff factor, i
The van't Hoff factor is close to the number of ions from a solute molecule, but it does depend on the extent of dissociation. Effectively it is the # of ions, for our purpose.
We can start with calculating the concentration in molality of NaCl. Since the mass of NaCl is 10% of water, we have 50.0g of NaCl. The molality of the NaCl solution is We apply the boiling point elevation expression Therefore, 1.8°C increase in temperature. So, the 10% solution of NaCl boils at 101.8°C.
Freezing Point Depression
For freezing point, the effect of having solution is in the opposite of boiling point, and the freezing point measured with sol'n is lower than the pure solvent.
where ΔT is the change in freezing point, m is the molality of sol'n, and kf is the freezing point depression constant. For water as solvent, kf is 1.86 °C kg/mol.
For ionic solute, the equation must be corrected by van't Hoff factor, i
We first need to convert 14°F to Celcius scale. Therefore, the ΔT = 10°C. We use this in . We solve for m (molality) with kf = 1.86 °C kg/mol. Since the capacity of the radiator is 10.0L, therefore, the mass is 10.0 kg, so this can be converted to ethylene glycol mass.
Consider also a question like the following:
Given the area of your yard and the height of snow on your yard, can you
calculate the amount of salt (KCl) required to melt all snow, if outside
temperature is 25°F and the density of the newly fallen snow is
0.15 g/cm3?
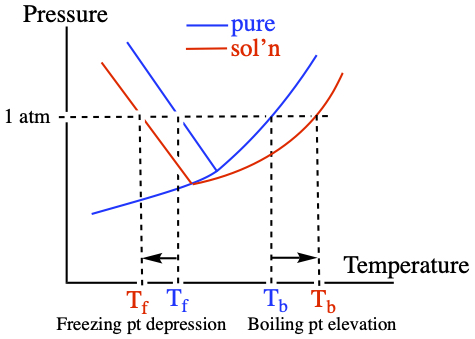
Pictorially, in terms of phase diagram, the freezing point depression and boiling point elevation are manifestation of the shift observed in the phase diagram, as shown below. The blue curve is the phase diagram for, say, pure water, and the red is for solution.
Osmotic Pressure
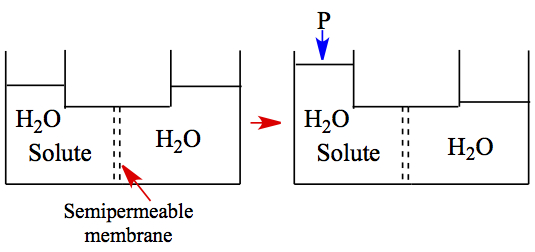 A bent tube, with a semipermeable membrane where only H2O molecules can pass,
contains a solution on the left chamber and pure H2O on the right. In order
for the system to reach equilibrium to equalize the concentration, water moves from the
right chamber to left. The pressure exerted on the left chamber to stop the flow of water
from the right is called osmotic pressure, π. The relationship between π and
concentration is as follows,
A bent tube, with a semipermeable membrane where only H2O molecules can pass,
contains a solution on the left chamber and pure H2O on the right. In order
for the system to reach equilibrium to equalize the concentration, water moves from the
right chamber to left. The pressure exerted on the left chamber to stop the flow of water
from the right is called osmotic pressure, π. The relationship between π and
concentration is as follows,
where M is the molarity of the solution. It resembles IGL!
Rearrange the equation so that
Π = 0.01974 atm,
so, n = [(0.01974 atmi)(0.01 L)]/(0.08206 Latm/molK)(293.15 K) = 8.2046 x 10-6 molprotein
and μ = 0.0500 g/(8.2046 x 10-6 mol) = 6094 g/mol
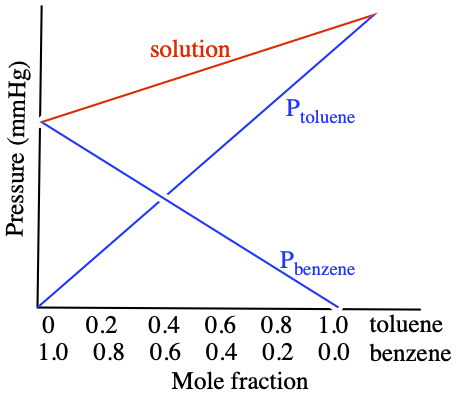
Fractional Distillation
From vapor pressure lowering for solutions with volatile solute,where subscripts 1 and 2 indicate solvent and solute that consists the solution, respectively. If you plot above equation in terms of a solution made of benzene and toluene in different composition, you might see something like this.
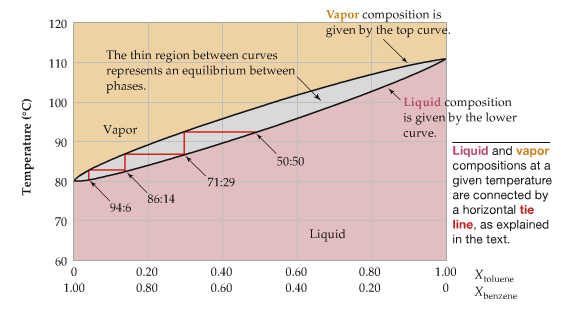
The diagrams shows the temperature phase diagram as a fxn of mole fraction.
The top part (orange shade) is gas phsae, and the bottom part (pink) is the liquid. The phase boundary in this diagram is separated, due to the volatile nature of the solute. It means that at a certain temperature, the vapor composition (mole fraction) is different from that of the liquid composition (mole fraction).
For example, at 90°C, vapor has 20% toluene, but liquid has 40% toluene. This difference is utilized to concentrate, for example, alcohol from a dilute solution.
So, we start with:
χalc = 0.05 : P°alc = 400 mmHg
χwater = 0.95 : P°water = 153 mmHg
Since P = P° χ, vapor pressures are
Palc = (400 mmHg)(0.05) = 20 mmHg
Pwater = (153 mmHg)(0.95) = 145.35 mmHg
Then, Ptotal = 165.35 mmHg So, now we can recompute the mole fraction in vapor by using χ = P/Ptotal
We have:
χalc = 20 mmHg/165.35 mmHg = 0.121
χwater = 145.35 mmHg/165.35 mmHg = 0.879
Now, you see alcohol content went up more than twice as much! If you condense this vapor to a liquid, and do the calculations over, you get:
2nd iteration
Palc = (400 mmHg)(0.121) = 48.4 mmHg
Pwater = (153 mmHg)(0.879) = 134.5 mmHg
Ptotal = 182.9 mmHg
χalc = 48.4 mmHg/182.9 mmHg = 0.265
χwater = 134.5 mmHg/182.9 mmHg = 0.735
3rd iteration
Palc = (400 mmHg)(0.265) = 106.0 mmHg
Pwater = (153 mmHg)(0.735) = 112.4 mmHg
Ptotal = 218.4 mmHg
χalc = 106.0 mmHg/218.4 mmHg = 0.485
χwater = 112.4 mmHg/218.4 mmHg = 0.515
4th iteration
Palc = (400 mmHg)(0.485) = 194.0 mmHg
Pwater = (153 mmHg)(0.515) = 78.8 mmHg
Ptotal = 272.8 mmHg
χalc = 194.0 mmHg/272.8 mmHg = 0.711
χwater = 78.8 mmHg/272.8 mmHg = 0.289
So, after 4 iterations, alcohol content is over 70%.
In actual experiment, the iterations are done naturally by setting up an apparatus such as shown below. You're going to use this set-up in Organic Chemistr next year!
By Original PNG by User:Quantockgoblin, SVG adaptation by User:Slashme - http://en.wikipedia.org/wiki/distillation, Public Domain, Link
Colloids
Colloid = dispersion of particles in another medium. There are several shown below:| dispersing medium | dispersed phase | name | example |
|---|---|---|---|
| gas | liquid | aerosol | fog |
| gas | solid | aerosol | smoke |
| liquid | gas | foam | whipped cream |
| liquid | liquid | emulsion | mayonnaise |
| liquid | solid | sol | milk of magnesia |
| solid | gas | foam | plastic foam |
| solid | liquid | gel | butter, jelly |
| solid | solid | solid sol | alloy |
Tyndall effect: Light scattering in colloids. Color that is scattered depends on the size of the suspended particles. --- important tool in pharmaceutical research, called dynamic light scattering. The following shows that the white light has been scattered in the colloidal suspension.

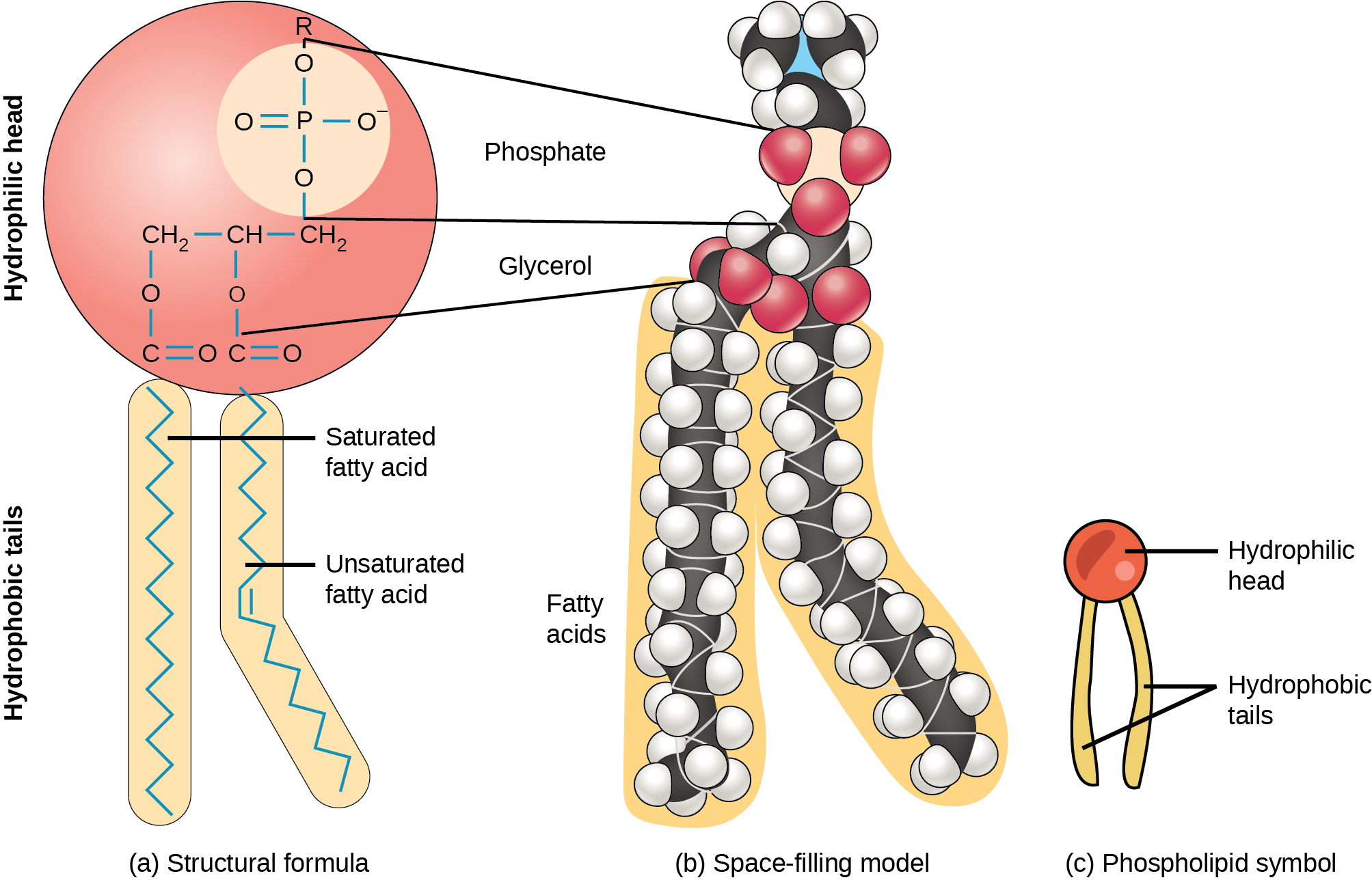
Hydrophilic and Hydrophobic Colloids
Two categories of colloids: 1) Hydrophilic and 2) Hydrophobic Example of hydrophilic colloids is protein in water. Hydrophobic colloids is not stable. For example, oil is not easily suspended in water.However, amphiphilic molecules can suspend large hydrophobic molecules in water. Amphiphiles are the molecules that possess both hydrophilicity and hydrophobicity within it. Biological cell is composed of phospholipids as you might know, as shown below. The assembly of such molecules make a cell membrane.