Dalton's Atomic Theory
Element — fundamental substance that can not be broken down chemically into simpler units.
Atomic Symbol — representation of elements in one or two-letter symbol
All are listed in the Periodic Table — Remember period and group from Chapter 1.
Dalton proposed:
Conservation of Mass
Matter can neither be created nor destroyed during a chem rxn. It also means that the mass of all reactants add up to the mass of all products.
Law of Definite Proportions
Compounds contain — same proportions of elements by mass
For example: Benzene has carbon and hydrogen and always 12g(C)/1g(H) ratio.
methane also contains C and H: 12g(C)/4g(H) = 3g(C)/ 1g(H)
Law of Multiple Proportions
Mass of compounds == Integral multiples of two or more elements
Example: If there is 17g of Ammonia, then there must be 14g of Nitrogen and 3g of Hydrogen. In ammonia, the ratio of H:N is always 1:4.632. Always.
Example: In a closed vessel, 0.455g of Mg is allowed to burn in O2 with its mass 2.315g. The unreacted O2 was found t be 2.015g. What is the mass of MgO2 produced?
Using Conservation of Mass, we know that:
Before Rxn: Mg(0.455g) + O2 → Total mass = 2.770g
After Rxn: O2(2.015g) + MgO( ? g) → Total mass = 2.770g
Therefore, m(MgO) = 2.770g - 2.015g = 0.775g MgO.
The Structure of the Atom
Electron
- In cathode ray tube (electron beam)
Thomson (1898): found negatively charged partilces in all atoms
Subsequent experiments determined me- ≈ mp+/2000
Devised an atomic structure to be "plum pudding"

The yellow sponge cake portion is the smeared positive charges
The electrons are embedded as chunks of plums, which oscillate back and forth.
... but this is a wrong picutre!
Radioactivity
Subsequently,
Radioactivity = spontaneous emission of particles or radiation
Proton and Nucleus
Applied α particles (from radioactive decay) to tink gold foil
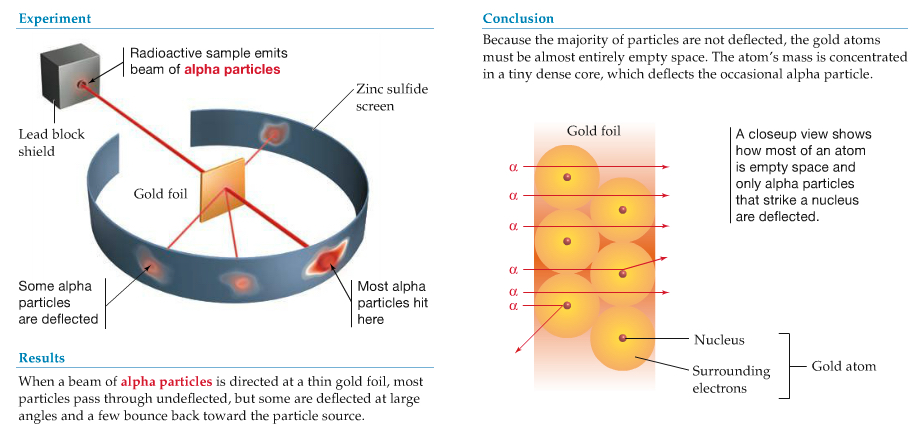
From this and other experiments, they concluded:
They proposed a "planetary model of the atom".
.... but this is also wrong!
Neutron
H structure was solved by Rutherford. But problem remained.Helium mass was 4 times as much as Hydrogen, for where Rutherford knew there is one proton. Helium is known for having 2 protons, then why 4 time as much mass?
Chadwick (1932, long after Rutherford's experiment of proton) found Neutron
Today's View
This is how we view today. more details in chapters 5 and 6.
Nuclear Components:
 |
| How atom should look |
Each of them is made up of 3 quarks.
It's just a fuzzy ball.
Today, this is how we view atom. A fuzzy ball. The blue halo is fast-moving electron cloud, and lighter the blue more electrons are found in that region. The halo effect comes from the fact that we can not pin point where the electrons are since they are in constant motion.
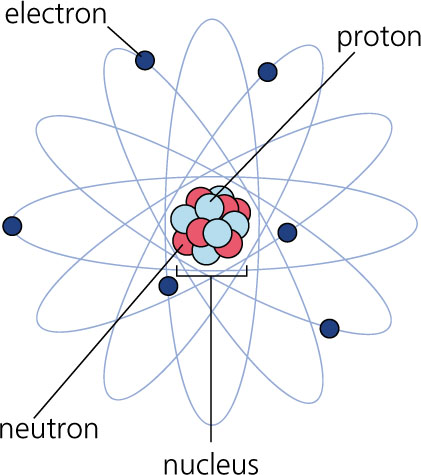
|
| Pictorial representation of atom |
This picture is much more familiar, but perhaps not very accurate. In the center, there is a nucleus, containing number of protons and nuetrons and surrounding the nucleus we have electrons moving.
Atomic Number, Mass Number, and Isotopes
In an atom
Define Mass # → A
Define # of protons → Z
Define # of neutrons → N
Then,
Z → differentiate atoms
If atoms share the same Z, but different N → Isotopes
A = Z + N
# of electrons = Z → therefore atom is electrically neutral
If # of electron in excess or deficient → Ion
and
If # of electrons > Z → Anion
If # of electrons < Z → Cation
For smaller atoms, the number of protons is the same as the number of neutrons. However, for larger atoms, the number of neutrons exceeds that of the protons.
Size Comparison: Baseball proton and baseball Sun Let's compare the size of Sun-Earth system with that of a simplest atom hydrogen, consisting only one proton and one electron. We're going to visualize them by making a baseball to either Sun or proton. The electron and Earth are represented by a smallest bead I could possibly find in my wife's hobby box! The following table gives the pertinent distances.
| Diameter | Distance | Diameter | ||
|---|---|---|---|---|
| Sun | 1.39 x 106 km | 1.5 x 108 km | Earth | 1.28 x 104 km |
| Proton | 1 fm | 0.53 Å | Electron | a point |
| Baseball | 7.5 cm | Small bead | 0.8 mm |
The ratio between diameters of Earth to Sun and small bead to a baseball is about the same. In both cases, the smaller one is about 1 % of the larger. Since electron is a point particle (to a good approximation), so there is no size. But, to visualize the system, we need to make electron a particle with finite size. The distance between the two parts of the systems are also listed.
From these we can calculate the distance from Sun to Earth if the size of the Sun is shrunk to that of a baseball.
To check the consistency of my baseball-bead hydrogen atom model, we use size of Earth (size of the bead) as our reference. If the following calculation agrees with the one we calculated with baseball (Sun), our model should be reliable.
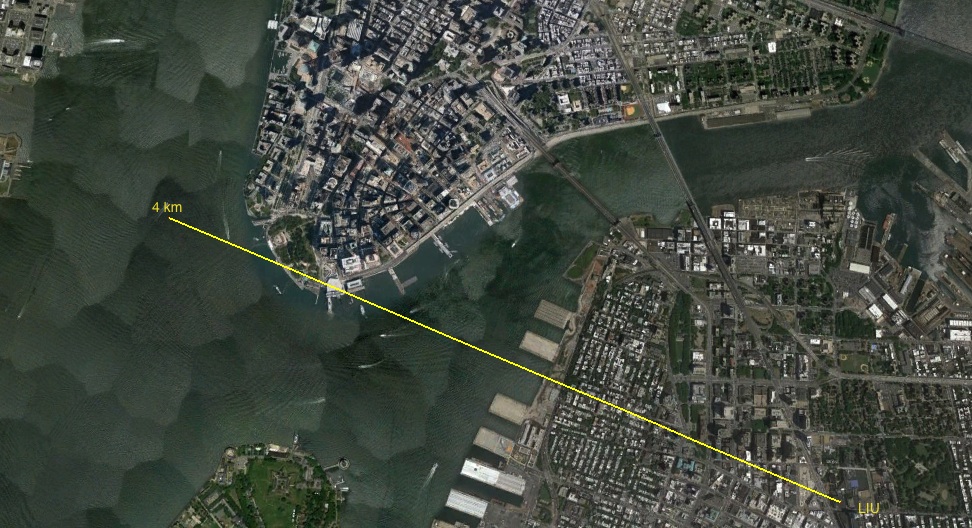
1 m or so difference. Not too bad. Two calculations are just consistent. Now, calculate for baseball hydrogen.
Wow!! So, if the baseball was our Sun, Earth would be orbiting only 8~9m away, but if the baseball was a proton in hydrogen atom, it would be zipping around near South Ferry in Manhattan!! (For those of you who are outside of LIU, we are located near Manhattan Bridge in Brooklyn, New York.)
Atomic Symbol
There are about 100 naturally occuring elements, listed on the Periodic Table.
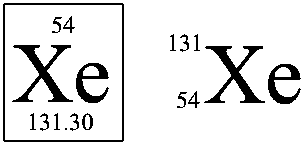
Representation on the left is from the Periodic Table and on the right is the isotopic notation.
In the above example, Xenon atom, with atomic symbol Xe, is given here.
The information we can obtain from these are,
Its A = 131 — from isotopic notation
The average mass <m> = 131.30 — from Perodic Table notation
From these information we can calculate:
# of electron = 54 = Z
Example: From 2.100. How many protons, neutrons, and electrons are in each of the following: a. b. c. d.
a. In this notation, the subscripted number, that is Z, is 7. It means that we have 7 protons. The superscripted number, A, is 15. It means that we have combined nuclear particles (protons and neutrons) counts 15. Then, the number of neutrons is N = A - Z and there are 8 neutrons. The number of electrons are the same as the number of protons in an atom, because atom is electrically neutral.
b. Similarly, Z = 27, N = 60 - 27 = 33, e- = 27
c. Z = 53, N = 131 - 53 = 78 , e- = 53
d. Z = 58, N = 148 - 58 = 90 , e- = 58
Atomic Mass
Atomic mass is represented by atomic mass unit (amu), and is defined by
where m(C-12) is the mass of carbon-12 isotope.
Using the above Xe example, hence, the average mass of Xe is 131.30 amu.
Example: How many atoms are there in 10.0 g of Na?
As we've seen in Chapter 1, we can use dimensional analysis to solve this problem. As you read the question, you can write the equation down as,
should suffice. The conversion of 1 amu is 1.660539 x 10-24 g.
That's a huge number!
The Periodic Table
Over 90 different naturally occuring elements listed in the Periodic Table of Elements

Group = elements in the same column
Period = elements in the same row
Each group has similar chemical properties
For example, Carbon belongs to group 14 so as silicon. Both can form up to 4 bonds.
Majority of elements are metals, i.e. conducts electricity and has shiny colors
| Group 1 | Alkali metals |
| Group 2 | Alkali Earth metals |
| Groups 3 - 12 | Transition metals |
| Groups 13 - 18 | Main group elements |
| Elements 57 - 71 | Lanthanides |
| Elements 89 - 103 | Actinides |
| Group 15 | Pnictogens |
| Group 16 | Chalcogens |
| Group 17 | Halogens |
| Group 18 | Noble gas |
Molecules and Ions
Molecule
Ions
Ion — Charged chemical compound.- If positively charged — Cation
- If negatively charged — Anion
Since atoms are electrically neutral, in comparison with the parent atom
Example: How many electrons are in the following atoms and ions? H, H+, Cl, Cl-, Mg2+, O2-
Remember that atoms are electrically neutral. It means that the number of protons, Z, is the same as the number of electrons. So, for H, the number of electron is 1. For Cl, it is 17. For cations, the number of electron is less than the number of protons. For H+, an electron with -1 charge is missiing from H, terefore, there is no electron, zero. For Cl-, it is 18, because when you add one -1 charge to 17 electron Cl atom, there will be an -1 charge overall, so there are 18 electrons in Cl-. Similar line of arguments will give you the number of electrons in Mg2+ to be 10, and O2- to be 10.
- Between cation and anion — there exits ionic force
- + charge and - charge attract each other
- If the ion is consist of more than one atom —
polyatomic ion
Table salt, sodium chloride (NaCl), is an ionic compound. It consists of Na+ cation and Cl- anion.
Vinegar is a solution of acetic acid and water. Acetic acid in water is partially ionic, consisting H+ ion and acetate ion (anion), as shown below.
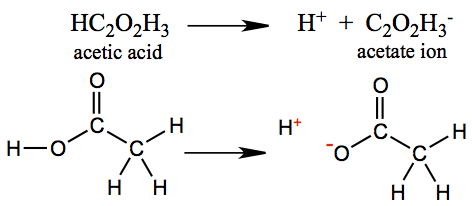
Phase of the compounds. You'll see often notation of chemical formula with its phase. NaCl(s) is solid phase of sodium chloride.
| phase | denotation | description |
|---|---|---|
| Gas | (g) | gas phase |
| Liquid | (l) | liquid |
| Solid | (s) | sold phase |
| Solution | (aq) | aqueous solution |
Chemical Formulas
Chemical formulas — representing the composition of molecules and ionsAtoms in a molecule are held by chemical bond.
Several different chemical formulas
- Structural formula — shows how atoms are connected to each other
- For example, chemical formula for benzene is C6H6
and the ratio between C and H is 1:1. So, empirical formula of
benzene is CH.
Below is an example of formulas for water.
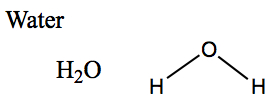
Chemical formulas and structural formula of water
The representation on the right is structural formula for describing the chemical bonding in the molecule. In the figure above, there are lines connected the oxygen atom to two hydrogen atoms. These lines represent chemical bonds.
There are two types of chemical bonds:
- Covalent bond—sharing two electrons between two or more nuclei
- Ionic bond—force associated with two or more opposite charged ions bond
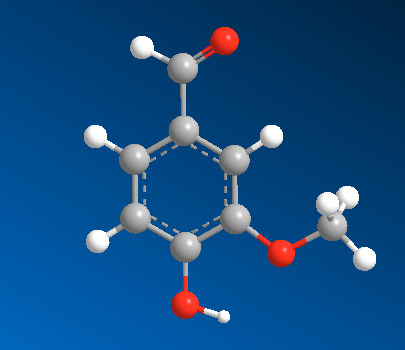
Here represented in four ways, vanillin molecule. This molecule is responsible for the very pleasant aroma of vanilla.
| Chemical Formula | Structural Formula | Organic Chemists | 3-D Ball-stick | 3d Space Filling |
|---|---|---|---|---|
| C8H8O3 | 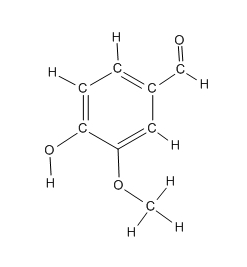 |
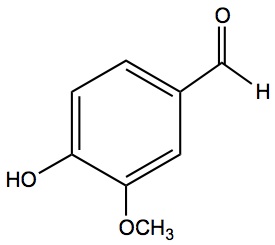 |
 |
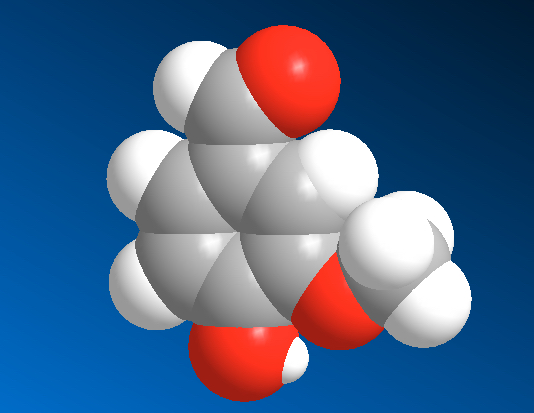 |
Two models on the left will be used in CHM3, but other noteworthy ones are also listed. The representation used by organic chemists (labeled as Organic Chemists) the one you learn in Organic Chemistry next year. The 3-D representation can be done in a number of ways. Here we list two, ball and stick model and space filling model.
Formula of Ionic Compounds
As shown above, table salt, NaCl, has ionic character. It can be separated into Na+ and Cl- ions. Again, between Na+ and Cl- ions, there exists the ionic force that holds these ions. Interaction between ions are:- Positive and Positive — Repulsive (repel one another)
- Negative and Negative — Repulsive (repel one another)
- Positive and Negative — Attractive (come together)
For example, magnesium bromide, where magnesium ion is Mg2+ and bromine ion (called bromide ion) is Br-, must be neutral, then there must be two Br- so that +2 charge on magnesium ion is cancelled. Thus, magnesium bromide is written MgBr2.
Nomenclature of Binary Ionic Compounds
Nomenclature — systematic naming scheme
Binary compound — made up of metal cation and main group anion
- Use atom name for cation
- For iron, Fe2+ = ferrous, Fe3+ = ferric
- Use —ide ending for anion
So sodium chloride is: NaCl. First one is cation Na+ and the latter is anion Cl-.
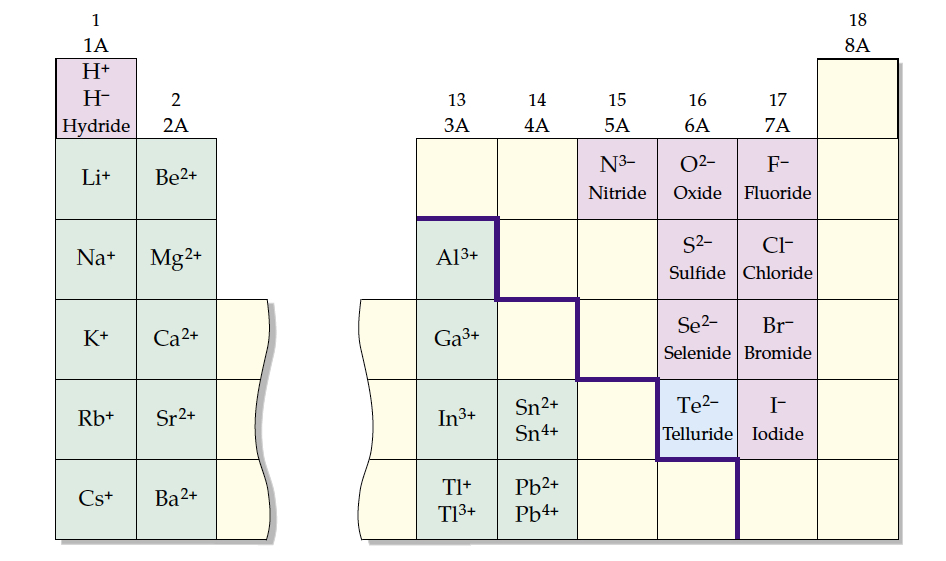
Example: Name the following compounds. LiCl, MgCl2, BeO, AlCl3, Al2O3
- LiCl = lithium chloride
- MgCl2 = magnesium chloride
- BeO = Berrium oxide
- AlCl3 = aluminum chloride
- Al2O3 = aluminum oxide
Example: Obtain the chemical formula for the following. Ferric chloride, Potassium telluride, Strontium bromide, Rubidium sulfide.
- Ferric chloride = FeCl3
- Potasium telluride = K2Te
- Strontium bromide = SrBr2
- Rubidium sulfide = Rb2S
When the compound contains some number of atoms, we need to know how many.
We use Greek-derived prefix for the purpose. For example,
TiO2
is commonly known as Titanium dioxide. Di- represents two. The following
table lists the prefixes.
| number | prefix | number | prefix | |
|---|---|---|---|---|
| 1 | mono | 11 | hendeca | |
| 2 | di | 12 | dodeca | |
| 3 | tri | 13 | triskadeca | |
| 4 | tetra | 14 | tetrakaideca | |
| 5 | penta | 15 | pentakaideca | |
| 6 | hexa | 16 | hexakaideca | |
| 7 | hepta | 17 | heptakaideca | |
| 8 | octa | 18 | octakaideca | |
| 9 | nona | 19 | nonakaideca | |
| 10 | deca | 20 | icosa |
Example: Give names of the following compounds. CO, CO2, N2O3, BrF3, S2F2
CO — Carbon monoxide
CO2 — Carbon dioxide
N2O3 — Dinitrogen trioxide
BrF3 — Bromine trifluoride
S2F2 — Disulfur difluoride
Naming with Polyatomic Ions
Somewhat preserved systematic naming in compounds with polyatomic ions. However, many of them use common names. Important ones that you encounter in this course are listed in the following table. Most of them are anions.
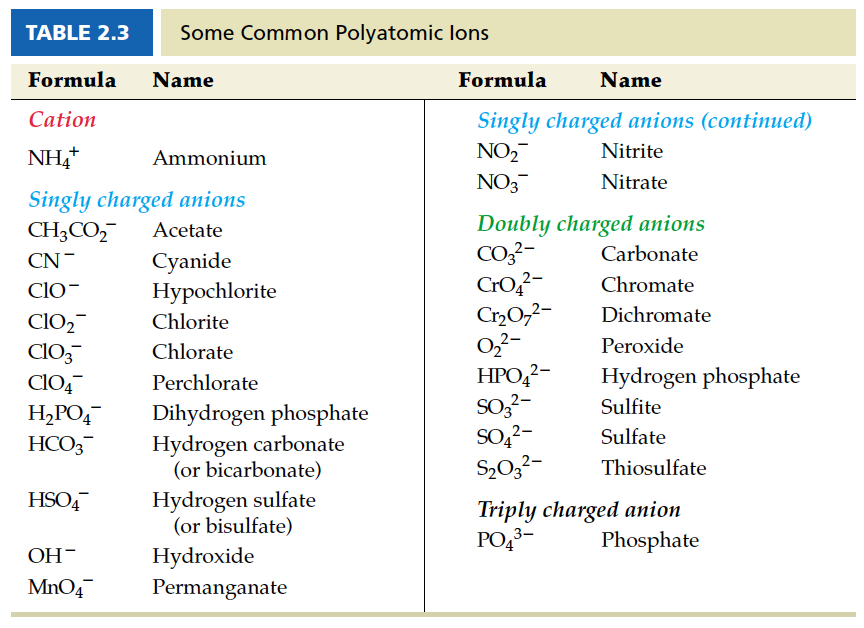
We have already talked about acetic acid above. Acetate ion is written as CH3CO2-. If acetate ion is combined with sodium ion, it would be called sodium acetate. Naming scheme is still the same.
Nomenclature of Acid As described above, acid produces H+ in aqueous solution. For example, hydrochloric acid is HCl in water, therefore, it is written as HCl(aq). This notation of adding (aq) and having dissociable H+ tells that the species is an acid.
Some of the common acids and anion produced from the acids are listed in the following table.
Example: Name the following compounds. HCl(g), HCl(aq), NaOH(s), KMnO4(s), HNO3(aq), Fe(ClO4)3(s), H2SO4(aq)
- HCl(g) — hydrogen chloride
- HCl(aq) — hydrochloric acid
- NaOH(s) — sodium hydroxide
- KMnO4(s) — Potassium permanganate
- HNO3(aq) — nitric acid
- Fe(ClO4)3(s) — ferric perchlorate
- H2SO4(aq) — sulfuric acid
Introduction to Organic Compounds
The names of hydrocarbon species one carbon to 10 carbons. You can rotate the molecules around.
| Name | Formula | Model |
|---|---|---|
| Methane | CH4 |
|
| Ethane | C2H6 |
|
| Propane | C3H8 |
|
| Butane | C3H8 |
|
| Pentane | C3H8 |
|
| Hexane | C6H14 |
|
| Heptane | C7H16 |
|
| Octane | C8H18 |
|
| Nonane | C9H20 |
|
| Decane | C10H22 |