Covalent Bond
When molecules are made, chemical bonds formed between different nuclei. The chemical bonds formed are so-called, covalent bonds. A covalent bond is formed between two nuclei so that resulting molecule is stabilizized, hence existence of the molecule.The interactions of particles in H2 molecule for example is only electromagnetic in nature, more specifically Coulomb interactions. As shown below, even a simple molecule such as H2 has this many interactions to be taken into account, although they are all Coulombic in nature,
where V is the interaction energy between two charged particles, ei and ej, rij is the distance between the charged particles i and j, and c is the proportionality constant. If the two charges are the same V > 0, which means that the interaction is repulsive. On the other hand, the two charges are different, V < 0, which means that the interaction is attractive. In H2, we have these interactions:
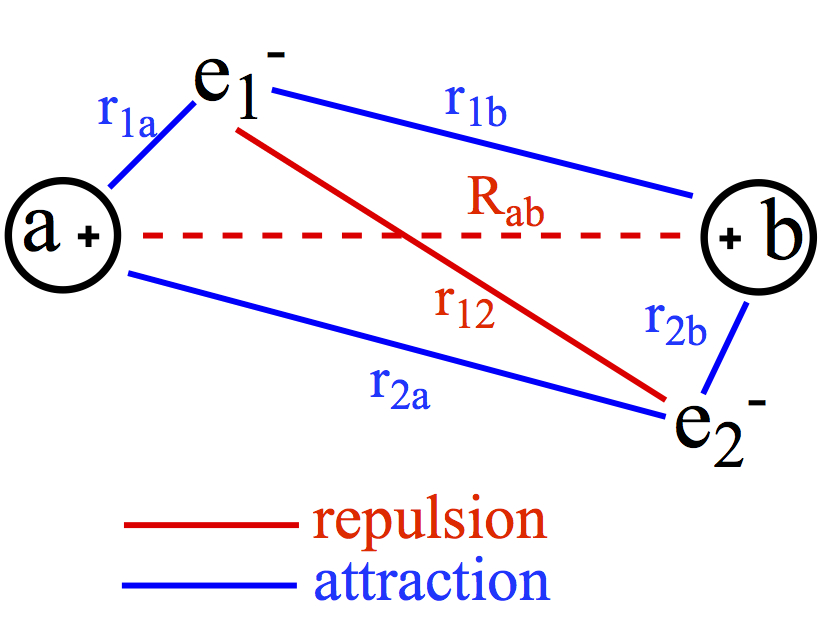
The red lines are repulsive interactions, and the blue lines represent attractive interaction. As simple as it seems, in H2 molecule has six interactions to worry about. When we put these interactions in Schrödinger equation, we obtain the quantum mechanical description of chemical bond.
Remember from the previous chapters that Schrödinger's theory is wave mechanics. So in order for two H atoms to form a chemical bond, two waves one from each H atom collide. The figure below shows what happens when the two hydrogen atoms collide. Notice the tails of the two wave functions overlapping.
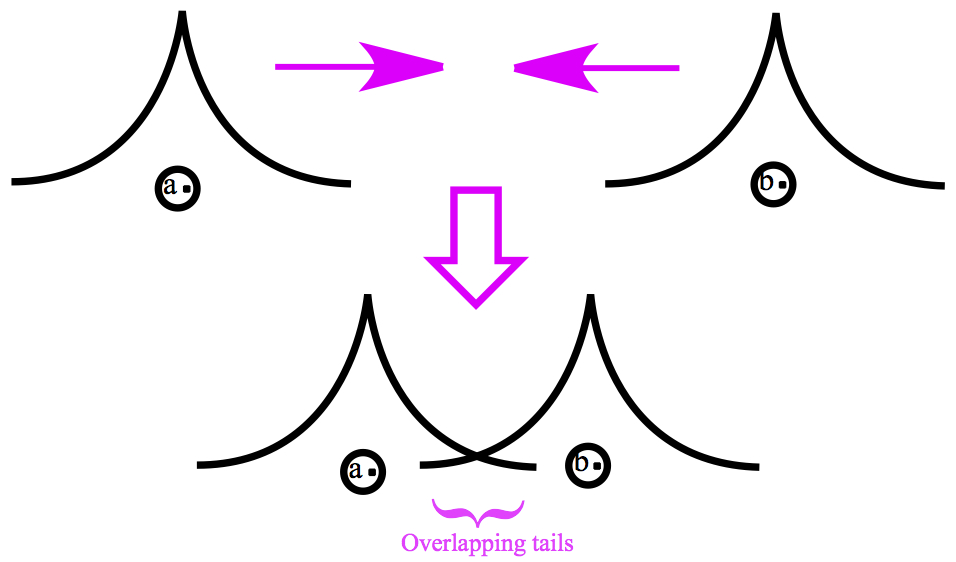
Afterall, these are waves. When, for example, two waves on water collide, the resulting wave shows interference pattern. Similarly, interference does occur in quantum mechanics, as well. Two types of interference: 1) constructive and 2)destructive. Constructive interference in H2 makes a bonding orbital, and destructive interference results in the so-called anti-bonding orbitals. (More on this later.) Basically, the bonding characteristic of covalent bond came from the interferences of two colliding wave functions.
As far as energy is concerned, when chemical bond forms, the molecule is stabilized. The stability of molecule, as compared to two separated atoms, tells that the energy is released when chemical bond forms. On the other hand, when you want a chemical bond to break, you need to inject energy into the bond. The relationship between energy of molecule and the bond distance between two nuclei is shown in the figure below.
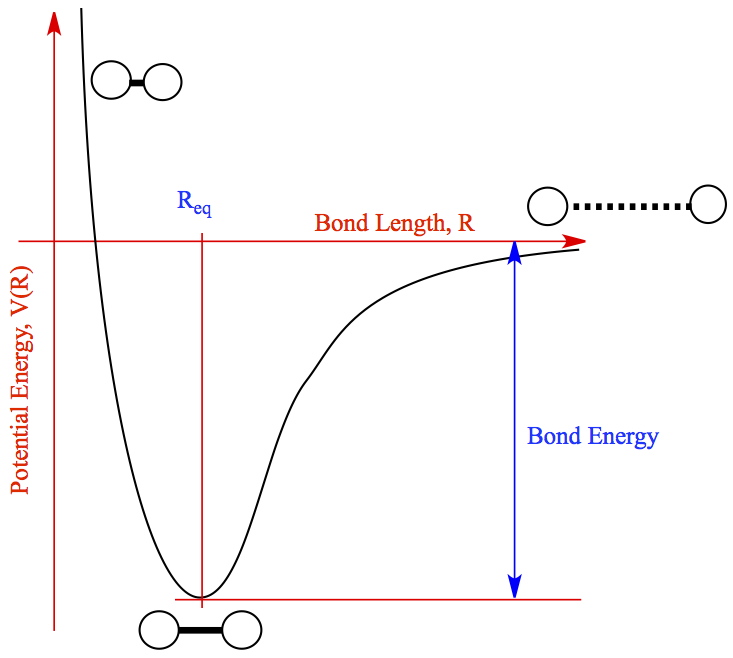
The general shape of the energy of any molecule as a function of bond distance looks like the above; different bonds have the different bond energy and the location of the equilibrium bond distance, Req, shown at the bottom of the well in the figure. The equilibrim bond distance is what we generally refer to as bond length or "bond distance".
So, from Req and try to break a covalent bond, you need to add energy to the system, while from two separated atoms to form a bond, there would be an energy release.
Strength of Covalent Bonds
It is possible for a molecule to have more than one covalent bond between the same nuclei. We generally refer to them as multiple bonds. If there are two, it is said that there is a double bond, triple bond for three, and so forth. For example, nitrogen molecule, N2, has a triple bond.

So, as shown in the figure above (energy of molecule as a fxn of bond distance), one needs to put energy into the molecule to break a chemical bond. If there is more than one bond between the two nuclei, it stands to reason that there are more energy needed to break the multiple bond. Here, some examples are presented.
| # of bonds | CC | NN | CO |
|---|---|---|---|
| Single | 350 | 240 | 350 |
| Double | 611 | 418 | 732 |
| Triple | 835 | 945 |
Polar Covalent Bonds: Electronegativity
When a chemical bond is formed, the electrons feel their environment. If the bond formation dealt between Na and Cl, due to Na's low ionization energy and Cl's high electron affinity, Cl grabs an electron from Na's valence shell to form Cl- ion and Na becomes Na+. In this case, the bonding is ionic. In the case of two carbon atoms, the resulting chemical bond is covalent in nature, and since both nuclei are C, the two C atoms share the two electron equally. However, there are many many molecules that their situations are in between NaCl and -CC- bondings. We refer to covalent bonding between the two extremes as polar covalent bond. A figure below shows the situation.
In the figure above, δ indicate a partial charge of atom in the molecule. The δ+ indicates that the atom has partiall positive charge, and the δ- indicates that the atom has partial negative charge. Even in a NaCl molecule, the overall charge of the molecule is zero, however, because of high electron affinity of Cl and the low ionization energy of Na, Cl gets - nad Na gets +. Similarly, in molecule in general has partial charges on atoms involved in bonding. One way to understand the polarity in covalent bonds, electronegativity can be used to assign partial charges on atoms. There are periodic trends in elelctronegativity, as shown in the figure below.
One can define electronegativity to be the ability of atom in a molecule to attract the shared electrons in a covalent bond. The highest electronegativity is assigned for fluorine, and the value of electronegativity decreases as going down the row in the periodic table, as well as going toward the left-side of the table.
-
If the electronegativity difference, ΔEN, between the two atoms
involved in the bonding is
- ΔEN > 2, then the bond is ionic
- ΔEN < 2, then the bond is polar covalent
One of the consequences of having a polar bond in a molecule is that, among other things, it can predict reactivity of molecules, particularly in organic chemistry that you take next year!
Electron-Dot Structures, Lewis Dot Structures
Covalent bond is to share two electrons between two nuclei. So, one can draw H2 molecule as the following.
The two dots in the middle indicate that two H's share two electrons. This is called electron-dot structure or more generally known as Lewis (dot) structure.
In order for us to draw a Lewis structure for a given formula, one must know the number of valence electrons. For the main group elements and hydrogen, we have:
| Group | # valence e- | Example | # of bonds |
|---|---|---|---|
| 13 | 3 | 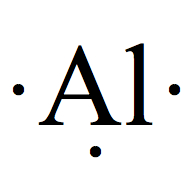 | 3 |
| 14 | 4 | 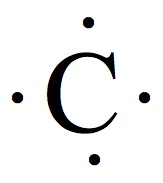 | 4 |
| 15 | 5 |  | 3 |
| 16 | 6 | 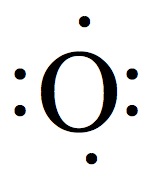 | 2 |
| 17 | 7 |  | 1 |
| H | 1 | 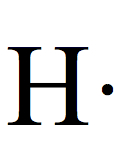 | 1 |
We now utilize the octet rule we learned in Chapter 6 to draw a Lewis structure. Let's use water as an example. For H2O, if we do the following all electrons are paired, and more importantly, oxgen atom satisfies octet.
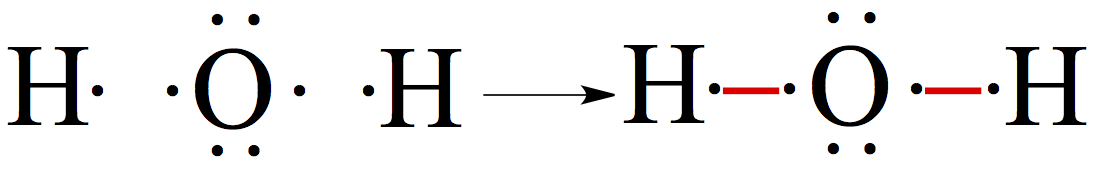
If we place the oxygen atom in the middle and two hydrogens on the side, you can connect two dots that are not paired (red lines). The line represents the covalent bond. Then, oxygen now satisfies octet, having 8 electrons around it.
You notice that the resulting water structure has two pairs electrons, these pairs of electrons are called lone pair. The lone pair electrons in general don't usually react to form a bond to another atom.
So, drawing a Lewis structure basically boils down to connecting two dots.
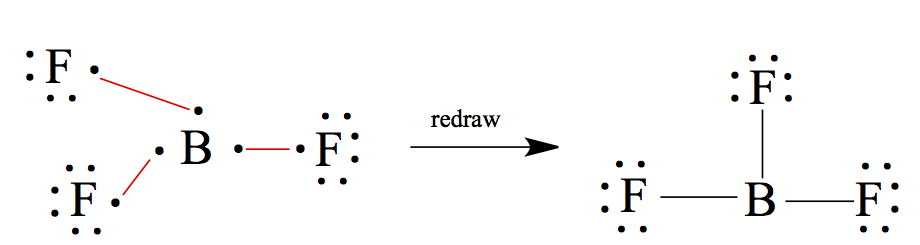
BF3: B is group 13, therefore 3 valence electrons, and F is group 17, thus 7 electrons. B should be the central atom.
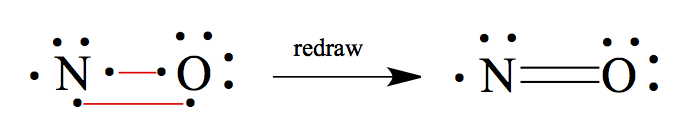
NO: N has 5 valence electrons and O has 6. So, connect two sets of unpaired electrons.
CO: Similar to above, but quite difficult case. Using what we have done, that results in the middle case, where we have a double bond. There are two other structures we can draw, without violating the octet rule.
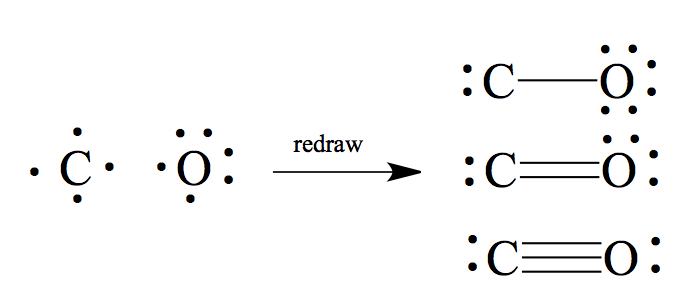
The actual winner is the triple bonded molecule. Generally, C-O single bond is about 1.43Å, and C=O double bond is 1.23Å. Carbon monoxide bond is much shorter, with 1.13Å, and therefore we must conclude that there must be a triple bond to be consistent with the bond length.
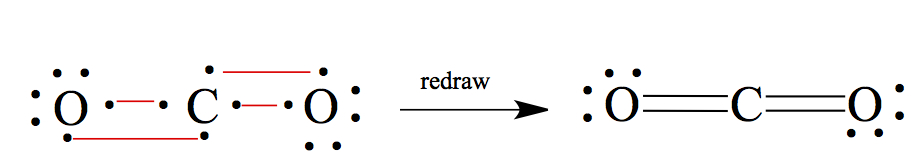
CO2: If you have more than two atoms, less electronegative element becomes the central atom, except for H. In this case, C should be in the center.
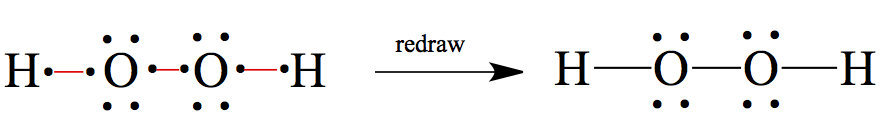
H2O2:
For larger molecules
There are many different structures one can draw for a given formula. For example, C3H4O, four different structures, called structural isomers, as shown below.
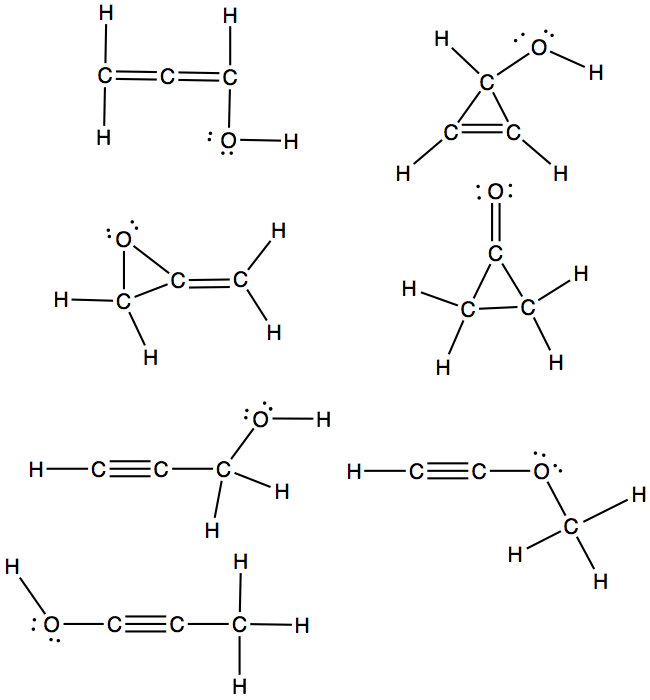
If the formula is C3H6O, then there are 7 isomers!! These are all application of connecting dots, and they all satisfy octet!
For molecules containing 3rd row elements and beyond
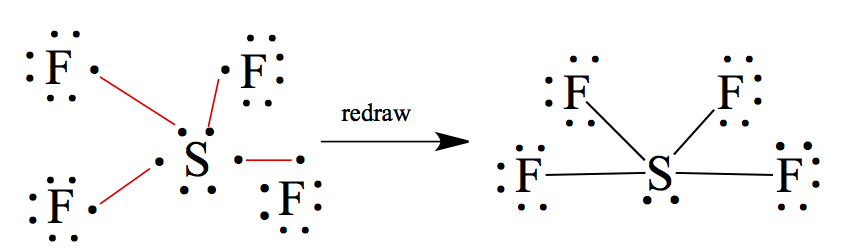
It is possible for 3rd row elements and heavier elements can have more than 8 electrons around the central atom. We call this expanded octet. For example, the structure of SF4 is shown below.
Resonance Structures
In the case of carbon monoxide structures we examined above, we said that between C and O there should be a triple bond, justifying our structure through experimental bond distance.Just by following the usual way of drawing Lewis structure, you should have arrived at the middle picture with a double bond. The two other structures can be deduced by writing resonance structures from the double bonded structure.
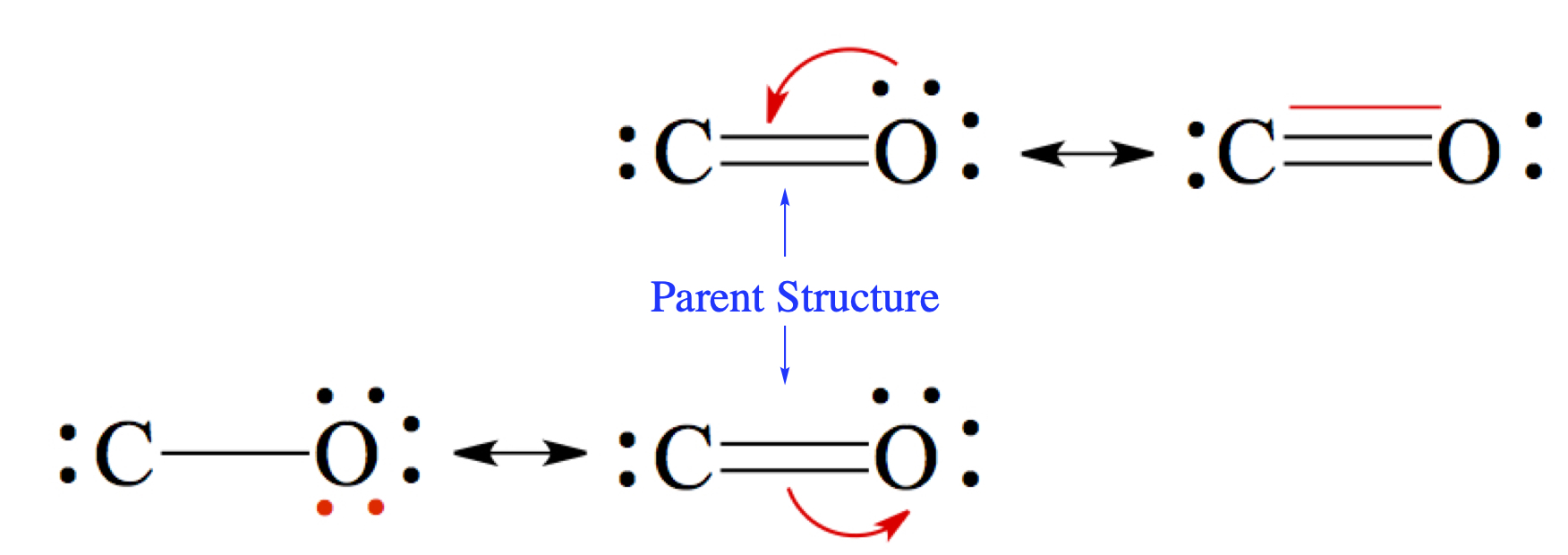
The double-sided arrow, ↔, in chemistry is reserved for resonance structures. The resonance structure with triple bond is constructed by taking one of the lone pair of oxygen and make the pair into a bond between C and O. You notice that there is no violation of the octet rule here. The bottom-left structure is make by moving a pair of electrons from the double bond to the oxygen. Here again, there is no violation of the octet rule. For the C atom, there are only four electrons, this makes C to have + charge and O to have - charge. In the triple-bonded molecule, the C atom has - charge, while O has +. See more in the next section.
Formal Charges
Again, I'd like to bring up the carbon monoxide example. We had single-bonded, double-bonded, and triple-bonded CO, and I made an excuse of having to compare the bond distance to say that the triply bonded CO is perhaps the best resonance structure. Often times, formal charge on the atoms in a molecule is calculated to differentiate the stability of particular isomer.Formal charge, Cf, is a fictitious charge of atoms in a molecule. One can calculate formal charge by using the following equation.
where nve, nbe, and nnb are the number of valence electros, number of bonded electrons, and number of non-bonding electrons, respectively.
Using the carbon monoxide example, we calculate the formal charges of C and O for each of the resonance structure.
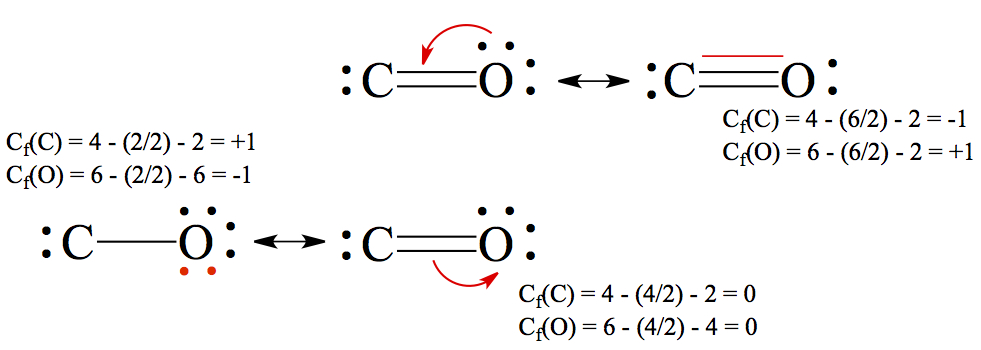
We can assess the stability of molecule by calculating the formal charges of atoms in the molecule, having more than one resonance structure.
This concept is important in Organic Chemistry!