Atomic Mass
Atomic mass is represented by atomic mass unit (amu), and is defined by
where m(C-12) is the mass of carbon-12 isotope.
If you look at Xe atom in the Periodic Table, forexample, the average mass of Xe is 131.29 amu.
Example: How many atoms are there in 10.0 g of Na?
As we've seen in Chapter 1, we can use dimensional analysis to solve this problem. As you read the question, you can write the equation down as,
should suffice. The conversion of 1 amu is 1.660539 x 10-24 g.
That's a huge number!
Moles
Remember the first example in this chapter? In a 17 g of ammonia, there are always 14 g of nitrogen and 3 g of hydrogen? Can we standardize the mass in terms of gram quantity because gram is the quatity we can directly measure? As amu is defined using C-12 as fundamental unit (for one atom) of mass, we can express atomic mass in grams in the macroscopic scale. For this we need to know the quantity (or number), for example, of nitrogen in 14 g.
We define Avogadro's number to be the number of C-12 atoms present in an exactly 12.0 g of C-12, and is 6.022 x 1023. This comes straight from the 1 amu = 1.660539 x 10-24g (Take the recipical of the number associated with g).
It is nuissance to write such a large number all the time: we must express 12.0g/6.022x1023 C-12. So we make this number into another unit; we call it a mole, abbreviated as mol. Therefore, 1 mol of substance always contains 6.022 x 1023 substances.
So, C-12 contains 6.022 x 1023 atoms, which is a mole of C-12.
Example: How many atoms are there in 12.0 g of N-15 and in 15.0 g of N-15?
We can solve this by dimensional analysis. One of the key conversion factors is relating C-12 to N-14. The number of atoms in 12 g N-15 is less than Avogadro's number—in C-12, however, it gives you Avogadro's number. For 15 g of N-15, the number of N-15 is the same as Avogadro's number. Hence, when the atomic mass in amu is the same as the gram quantity, you have 1 mol.
Average Mass
The average mass comes from averaging over all isotopic masses.
- (Isotope = has the same # of protons, but differing in # of neutrons)
If you add all the c1 + c2 + c3 + c4 + … = 1
Here an easy example of average mass calculation.
Example: Copper has two naturally occuring isotopes. Cu-63 with its mass = 62.94 amu and its abundance = 69.17%, and Cu-65 with its mass = 64.93 amu and its abundance = 30.83%. What is the average mass in amu?
We have,
to which we know all terms on the right-hand side of the equation. Only thing you need to worry about is the abundance expressed in percentage. The fractional abundance is obtained by diving the % by 100. Then,
Above is rather too easy. What about the following?
Example: Copper has two naturally occuring isotopes. Cu-63 with its mass = 62.94 amu and Cu-65 with its mass = 64.93 amu. If the average mass of copper is 63.55 amu, what are the natural abundances in percentage of each isotope?
This time, there are two unknowns, both the c63 and
c65. One equation with two unkowns can not be solved.
Nonetheless, we have again
but we also have
Using this, we now have two equations with two unknowns. This is solvable.
We can rearrange the second equation above to sove for c63,
then substitute the c63 equation into the average mass
equation (1st eqn) to obtain
Once you get the c65 isolated, you can put all the numbers in. Then, you get,
Therefore, c65 = 30.65%. Then, c63 = 69.35%. Comparing with the example above, the answer is reasonable.
Molar Mass
So the mystery of where the fraction in the average mass came from, is solved. Let's go back to mole quantity. The average mass listed on the Periodic Table, in the case of Xe, was 131.30 amu. It is much more covenient for us to express this in macroscopic quantity. The mole and amu are defined through C-12, 12 g is 1 mol, and we saw above that 15 g of 15 amu N-15 gives you 1 mol. Therefore, atomic mass in amu gives the equivalent number in g, and always gives the Avogadro's number amount. The average mass works exactly the same way. The average mass of Xe is 131.30 amu, therefore in 131.30 g gives 1 mole. This is called molar mass of element. The unit is g/mol.
Example: What are the molar masses of He, Ca, and Cu?
Just look at the Periodic Table and read off the average masses. For He, it is 4.003, therefore 4.003 g/mol. For Ca, it is 40.078, therefore 40.078 g/mol. Cu = 63.546 g/mol.
Molar Mass of Molecules
The average masses of atoms on the periodic table give the grams of element in one mole of that element. We call it, molar mass of element.
For molecules, molar mass, denoted as μ (mu of Greek character), is given as the total mass of the molecule in one mole of that molecule. Therefore, for water molecule,
Example: Molar mass of water:

Not much more complicated example...
Example: Molar mass of Prussian Blue (indigo dye):
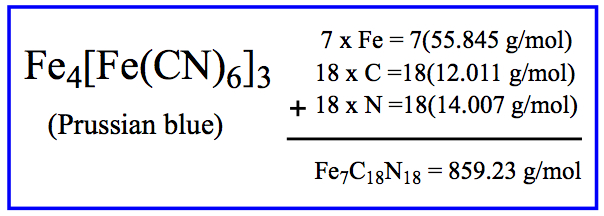
Chemical Calculations
Now you're in the position to perform chemical calculations. For this purpose, we follow dimensional analysis! What's important here is that when comparing two chemical species, you must use stoichiometry, and hence you need to have the balanced chemical equation.
Let's start simple.
Example: a) How many moles are there in a 10.00g of CO2? b) How many g are there in a 10.0 mol of CO2?
The molar mass of CO2 is 44.01 g/mol. So, we ask: a) for calculating the number of moles is
The second part is similar.
g to mol and mol to g → always done with the molar mass
Percent Composition
Percent composition of elements in a molecule: We can use % by mass to obtain the percentage of elements in compounds.
In water, 11.19% is H, and 88.81 is O. It then means that for a 100 g of H2O, there are 11.19 g H and 88.81 g O.
Example: Mass Percent
What is the mass % composition of H and O in H2O?
Since there are 2 mol H and 1 mol O for 1 mol of H2 with
Example: Empirical formula from % composition
Using the above % for H2O, obtain simplest formula (empirical formula).
So, in 100 g H2O, there are 88.81 g O and 11.19 g H. Since the chemical formula canb be thought as the numerical mole ratio of elements. Let's convert the mass of each element to # of moles.
Molecular formula is simple numerical ratio between elements in molecules. So, 11 mol H to 5.6 mol O reduces to 2 mol H and 1 mol O. This is why water has formula H2O.
Basically, by writing down the number of moles calculated from % composition for each element, then divide the numbers by the smallest value of moles. Here the important thing it to calculate the number of moles from %.
Chemical Rxn
Chemical reaction is represented by chemical equation, which separates reactant(s), which is starting material(s), to products(s), which is end result(s) of the reaction, by using an arrow (→), representing the direction to which the reaction proceeds.
Example: Given the following chemcial equation, calculate various quatities.
Let's start with:
How many moles of Cl2 do you need to obtain 2 moles of
PCl3?
How many moles of PCl3 can it be obtained if you have 2.0 mol of P4?
If you have 2.00 g of P4, how many mole of PCl3 can it be obtained?
If you have 2.00 g of P4, how many grams of PCl3 can it be obtained?
So, above examples shows the complexity of converting grams of cetrain reactant to grams of certain product is easily calculated.
Experimental Determination of Empirical Formula
Using the procedure to obtain empirical formula from % composition, we can work out the so-called combustion analysis. Combustion of hydrocarbon, species that contains only C and H, is a process where the hyrdocarbon molecules react with O2 to produce carbon dioxide and water.
So, if you know the amount of CO2 and H2O produced in the combustion process, we can calculate the values of x and y, and therefore we can obtain empirical formula of the reactant.
Example: Combustion Analysis
Combustion of hydrocarbon produced 33.01 g CO2 and 13.51 g H2O. What is the empirical formula of the hydrocarbon?
From equation above, the number of moles of C in CO2 came from CxHy, so as the number of moles of H in H2O. If we covert the mass of CO2 to mol of C, and the mass of H2O to mol of H, we can obtain the empirical formula for the hydrocarbon.
Above calculations show C0.750H1.50, and yields CH2 by dividing both numbers by 0.750.
Example: Combustion Analysis #2
A 12.01 g tartaric acid contains C, H, and O. Upon combustion, 14.08 g CO2 and 4.32 g H2O are produced. Deduce the empirical formula.
Now we have added complication of having O in the compound. Oxygen can come from O2 and these oxygen becomes distributed into CO2 and H2O, so we must determine the mass of O in the sample somehow.
The way to calculate the mass of O in the sample is to calculate the masses of C and H and subtracted from the total sample mass (12.01 g). Then, you have the mass of oxygen from the original sample.
If one calculates the molar mass for the empirical formula, you get 74.046 g/mol. The actual molar mass of tartaric acid is 148.092 g/mol. Therefore, if the empirical formula is multiplied by two, one gets the correct formula.
Balancing Chemical Equation
Chemical Equation
Chemical equation shows the transformation of molecules starting with the
reactants, the left-hand side of the equation, and ending
in products on the right-hand side. The arrow separates the reactants
from the products. Below is an example of a reaction for water molecules
transformed into hydrogen molecules and oxygen molecules.
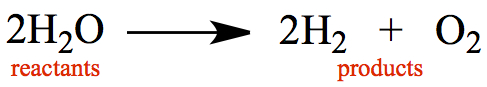
From the point of view of Conservation of Mass, it stands to reason that all atoms present in the reactants have to be present in the products.
There are 4 hydrogen atoms and 2 oxygen atoms on the left-hand side.
There are 4 hydrogen atoms and 2 oxygen atoms on the right-hand side.
When the chemical equation satisfys mass conservation, it is said to be balanced.
Balancing
Some tips upon balancing chemical eqns.
Example: Balance the following:
Since on the right-hand side, there are two Al and three S atoms, the left-hand side must have respectively the same Al and S atoms. So,
Too easy? What about next one?
Example: Balance the following:
Step by step.
First to match the number of C
We need at least 8 H, so
By doing above, you realize you have 8 oxygen on the right-hand side, but only 4 on the left, so
Finally, adjust N by,
However I explain it, you have to practice to get a hung of it!
Stoichiometry
Consider the following:
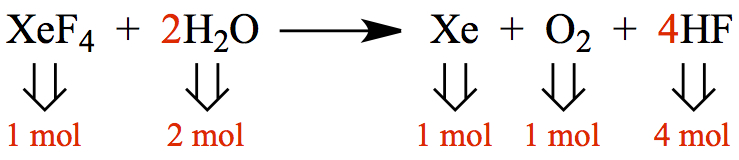
We can pictorially represent above chemical eqn as,
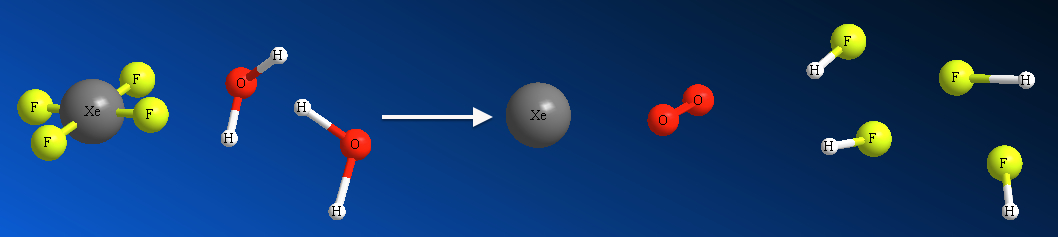
This is a microscopic view with each of the molecules in the reaction are shown.
How should we represent this microscopic knowledge onto the macroscopic view? What I mean by is, say, you are asked to calculate the gram quantity of XeF4 to make 10.0 g of HF. Now, it is no longer a single-molecule picture. Remember that a mere 18.0 g of water contains 6.022 x 1023 molecules!!??, i.e. 602 200 000 000 000 000 000 000 of them!!!!
For macroscopic quantities, we define mole, abbreviated as mol.
It means that there are 12.011 g of naturally occuring carbon, containing different isotopes. It means that the average masses of the elements on the Periodic Table are the masses of each element in 1 mol of that element.
Therefore, the number of molecules in the microscopic picture also corresponds to the amount of moles of each molecules. The coefficients in front of chemical formula are so-called stoichiometric coefficients. They represent the number of moles!
Yield of Product
Acutal vs. Theoretical Yields
The amount of product actually produced is called yield (or actual yield). Since the reaction of interest may have other pathways so that the the product may not form as much as it should, or even your filteration system may have reduced your products. So, the actual yield may be lower than the theoretically possible amount of product. The theoretical yield is the maximum amount allowed by the stoichiometry.
Percent Yield
Example: % yield of reaction:
Given the following reaction.
%yield can be used as the conversion factor in the dimensional analysis!
It means that some # of gsomething actual = 100 gsomething theoretical
Limiting Reactant
Here is a recipe for pizza:
| Item | Qty |
| Dough | 1 |
| Tomato sauce | 5 oz |
| Cheese | 2 cups |
So, if you have above quantity of items, you can make a pizza. If you have two doughs, but you still have a 5-oz tomato sauce, and 4 cups of cheese, how many complete pizza can you make? Obviously only one still, because of the 5 oz tomato sauce. In chemical terms, the equivalent of tomato sauce here is what we call limiting reactant, and this yield only one pizza.
Example: Limiting Reactant: What is the mass of PCl3 formed from 125 g of P4 and 323 g of Cl2 in the following reaction?
Therefore, Cl2 yields smaller quantity of product. Hence, Cl2 is the limiting reactant, yielding 417 g.