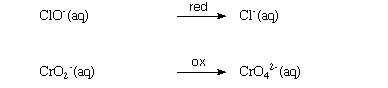
Using the above example, the half-reactions are balanced in the following
way using the table above.
| Reduction Reaction | Oxidation Reaction |
|
Step 1 -- There is only Cl atom to adjust. The left-hand side contains
one Cl. The right-hand contains one Cl, and therefore they are balanced.
|
Step 1 -- There is only one Cr atom on the both sides of the equation.
Therefore, it is balanced in terms of atoms other than O and H.
|
|
Step 2 -- By adding one H2O to the right-hand side of the
eqution make Oxygen atoms balanced.
|
Step 2 -- On the right-hand side, there are two Oxygen atoms more than
those of the left-hand side. So, add two H2O to the left.
|
|
Step 3 -- Since two Hydrogens are introduced in Step 2, 2H+
must be added to the left-hand side of the equation.
|
Step 3 -- Since two H2O (4 H's) are added at Step 2, four
H+ must be added to the right-hand side.
|
|
Step 4 -- Overall charge on the right-hand side is -1 due to the charge
on the Cl- ion. The left-hand side has +1 before adding
e-. In order to make the charge on the left-hand side to
come out to be the same as the right is to add 2e- on the left.
|
Step 4 -- The charge on the left-hand side is -1. The righ-hand side
is +2. Therefore, one should add three e- on the right-hand side
to match the overall charges on the both side.
|