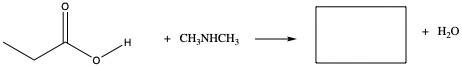Alkanes
Nomenclature
Systematic naming scheme for organic compounds -- IUPAC rules- Name the parent chain
- Name the alkyl groups attached to the parent chain
- Determine the point of attachment of alkyl groups to the parent chain
- Alphabetical order the alkyl groups with numbering specifying the position
- choose the longest chain as the parent chain
- You can check the table below for the names of alkyl groups
- Number the parent chain such that the branch alkyl group is attached to
the smaller numbered carbon.
| # of C | Name | Group Formula |
|---|---|---|
| 1 | methyl | 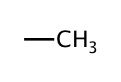 |
| 2 | ethyl | 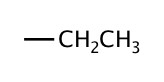 |
| 3 | propyl | 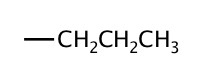 |
| 4 | butyl | 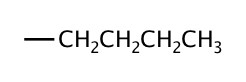 |
| 5 | pentyl | 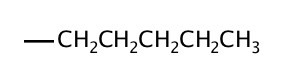 |
| 6 | hexyl | 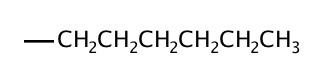 |
| branched groups | ||
| 3 | isopropyl | 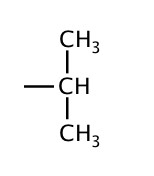 |
| 4 | isobutyl | 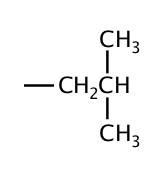 |
| 4 | sec-butyl or s-butyl | 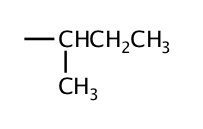 |
| 4 | tert-butyl or t-butyl | 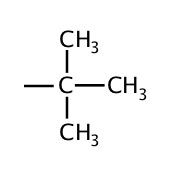 |
When naming:
- alkane --- replace the -yl of each of the group with -ane
- alkene --- replace the -yl of each of the group with -ene
- alkyne --- replace the -yl of each of the group with -yne
 , and six-carbon is hexane
, and six-carbon is hexane
 .
.
Four-carbon chain hydrocarbon with a double bond is butene
 . Both are butene; notice the
position of the double bond.
One can have two double bonds with 4-carbon chain
. Both are butene; notice the
position of the double bond.
One can have two double bonds with 4-carbon chain
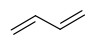 , we call this molecule
butadiene..
, we call this molecule
butadiene..
Five-carbon chain with a triple bond is pentyne,
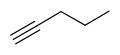 .
Also, different triple bond configurations would also be pentyne,
.
Also, different triple bond configurations would also be pentyne,
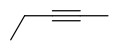 .
.
Example: Name the folowing molecules.
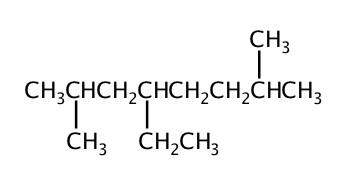
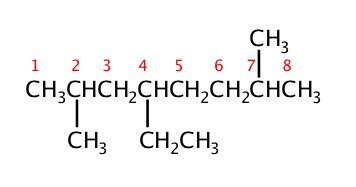
Constitutional Isomers
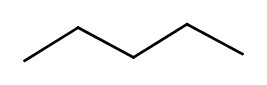
When chemical formulas are the same, but atomic connections are different, such molecules are called constitutional isomers.
So, for example, the following two melecules are the constitutional isomers of C5H12:
 |
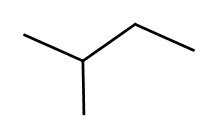 |
| pentane | 2-methylbutane |
Conformations
We learned that you can assign 3-dimensional shape associated with carbon atoms depending on the bonding situations. If the molecules have the same formula and the atomic connections are the same, but they differ in shape, is called conformers. For example, the following two structures are both butanes, but the have different conformations.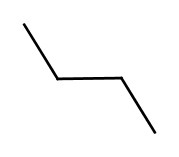 |
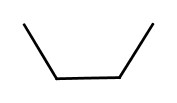 |
| These two structures are converted back and forth with nearly free rotations. | |
Cycloalkanes
As we have seen in Chapter 4, there are many different structures we can make from a chemical formula. One of such was cyclic structures. In the following table, some cycloalkanes are listed:| Name | Lewis Structure | Skeletal Structure | Side view |
|---|---|---|---|
| cyclopropane | 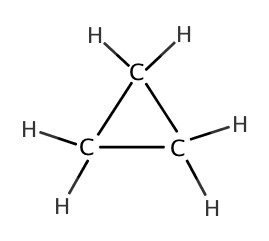 |
 |
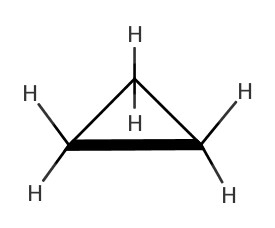 |
| cyclobutane |  |
 |
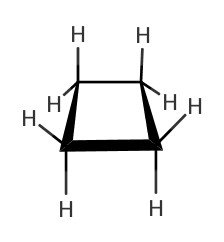 |
| cyclopentane | 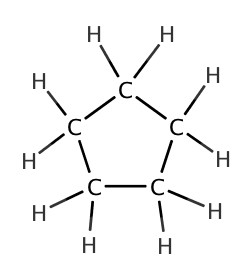 |
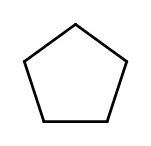 |
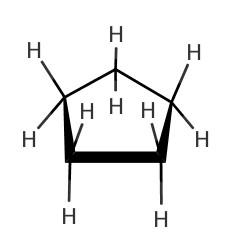 |
| cyclohexane | 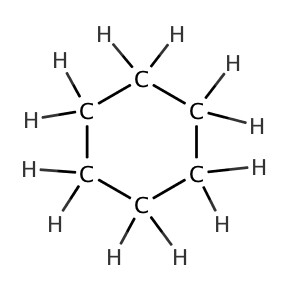 |
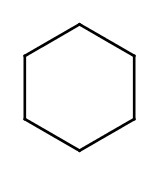 |
 |
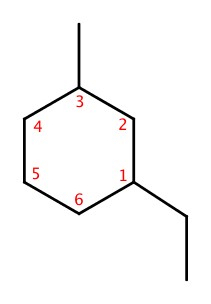
IUPAC names of cyclic alkane is to have the same rule:
Alkenes, Alkynes, and Aromatic Compounds
Alkenes contain at least one double bond. Alkynes contain at least one triple bond.Naming
Naming of alkene and alkyne is given such that the double bonded (or triply bonded) carbon gets prioritized. For example,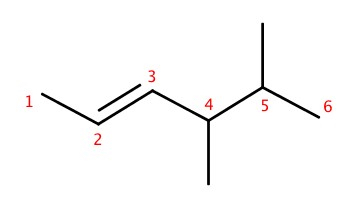
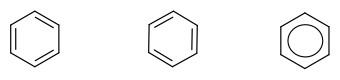
Aromatic ring is special cyclic alkene. Because of three double bonds, benzene is a very stable compound. The two compounds on the left side are equivalent. So, we generally combine them together to draw benzene to be what is represented on the right-most structure with a circle.
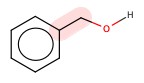
Benzyl alcohol
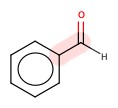
Benzaldehyde, the smell of almonds
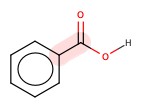
Benzoic acid
Geometric Isomers
When there is a double bond, the rotation along the C-C bond is no longer a free rotation. This creates two distinct geometric isomers. For example,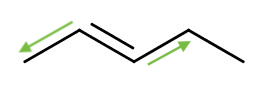
| 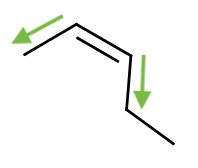 |
| trans-2-pentene | cis-2-pentene |
| Notice the orientation of the functional groups along the double bond. | |
Reactions of Hydrocarbons
Alkanes
Halogenation
You can add halogen atom to alkane by using light, symbolized by . There is no specificity as to where the chlorine atom attaches.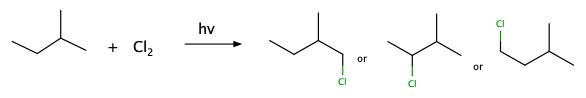
Alkenes and Alkynes
Pt catalysis: hydrogenation of alkenes and alkynes
We've seen this in Chapter 4.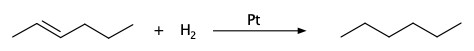
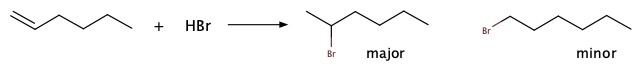
Markovnikov's rxn: HCl or HBr addition across double bonds
Alkenes and alkynes can be halogenated as well as hydration via Markonikov mechanism of protonation. The first step is to add H+, and this step is crucial in product distribution. The cation formed upon addition of H+ makes a cation on some carbon centered at less hydrogen attached in the original compounds.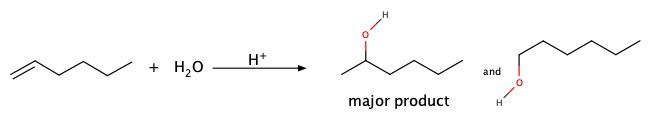
Halogenation on benzene:
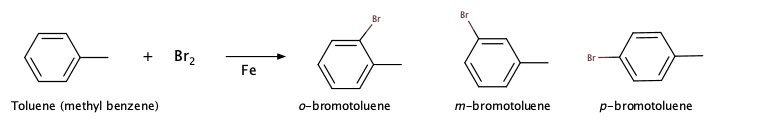
The following reaction is called a class of reaction, aromatic substitution reaction.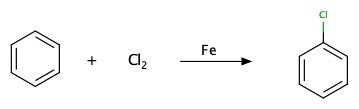
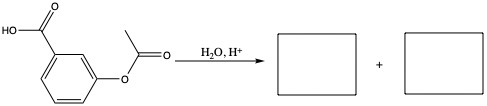
Carboxylic Acids
Naming of carboxylic acid is similar to what we have seen so far. For example, the following compound,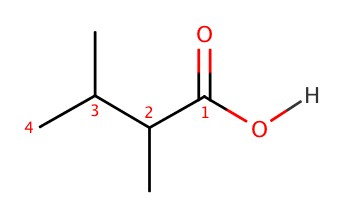
Phenols
The structure of phenol is as follows: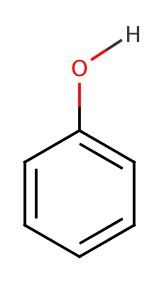
Caroxylic Acids and Phenols as Weak Acids
Weak Acid = acid that doesn't dissociate completely. And, weak acid forms an equilibrium,We have seen that acetic acid is a weak acid,
Phenol is weaker acid, and is given by the following:
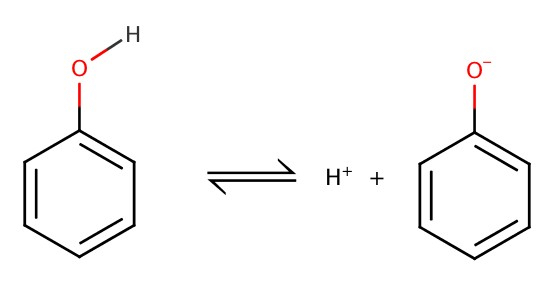 Ka = 1.1 X
10-10 or pKa = 9.96
Ka = 1.1 X
10-10 or pKa = 9.96
Salts of weak organic acids are used as perservatives in the food industry. You've seen some of those in the list below:
| Name | Structure | Parent Acid |
|---|---|---|
| Sodium benzoate | 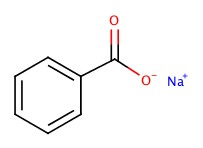 |
Benzoic acid |
| Potassium sorbate | 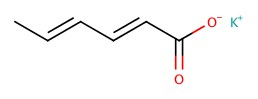 |
Sorbic acid (hexa-2,4,dienoic acid is correct) or even 2,4-hexadienoic acid |
| Potassium p-bromophenoxide | 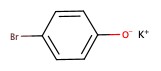 |
p-bromophenol |
Biological carboxylic acid
Depending on pH, the structure changes: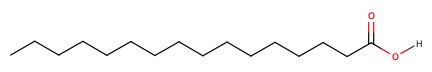 |
pKa = 4.75 | pH > 5 --deprotonated pH < 5 -- protonated |
| Palmitic acid |
Acids used for chemical exfoliation: All are α hydroxy acids.
| Formula | name | source | pKa |
|---|---|---|---|
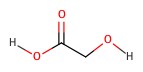 | Glycolic acid | sugarcane | 3.83 |
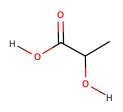 | Lactic acid | sour milk | 3.08 |
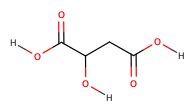 | Malic acid | apples and grapes | 3.40 |
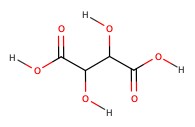 | Tartaric acid | wine | 2.98 |
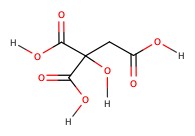 | Citric acid | citrus | 3.18 |
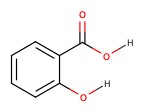 | Salicylic acid | willow bark | 2.97 |
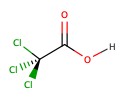 |
Trichloroacetic acid | chemical synthesis | 0.70 |
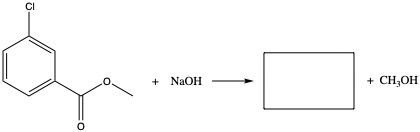
Preparing Esters
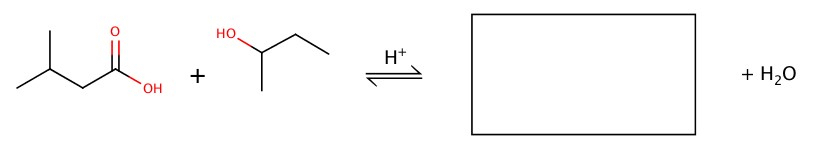
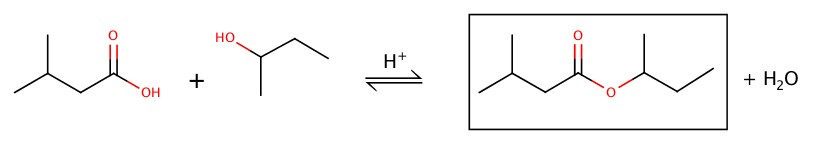
Preparation of ester is done by adding carboxylic acid and alcohol with a help of acid.
Example: Fill in the blank.
Amines
1° (primary) amine - one carbon next to N2° (secondary) amine - two carbons directly attacehed to N
3° (tertiary) amine - three carbons directly attacehed to N
4° (quaternary) amine - four carbons directly attacehed to N
Naming is done by the following way:
| Type | Name | Formula |
|---|---|---|
| 1° | Ethanamine | 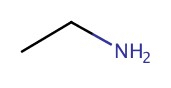 |
| 1° | propanamine | 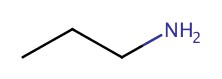 |
| 1° | butanamine | 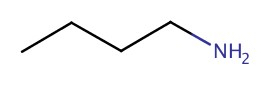 |
| 1° | pentanamine | 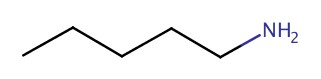 |
| 2° | N-methyl-1-propanamine | 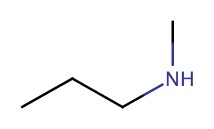 |
| 2° | N-ethyl-1-propanamine | 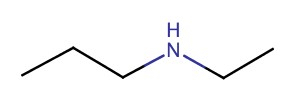 |
| 2° | N-methyl-1-propanamine |  |
| 2° | N-methyl-1-butanamine | 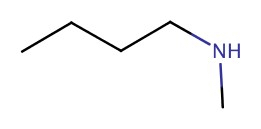 |
| 2° | N-methyl-1-pentanamine | 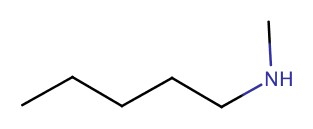 |
Cyclic amines are very important in biological chemistry.
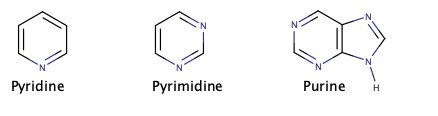
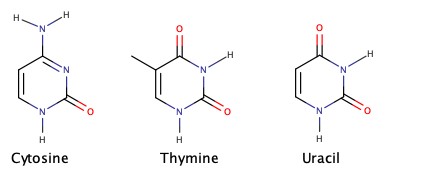
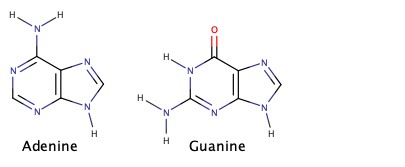
Amines as Weak Base
Amine is a weak base. The amine nitrogen has a lone-pair electrons, which makes amine to accept H+. This is why amine is a weak base.
For example, methylamine, CH3NH2 form an equilibrium,
The pKa of aniline is 4.6. If pH is below 4.6, then the aniline is protonated, while pH > 4.6, aniline is unprotonated.
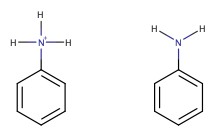
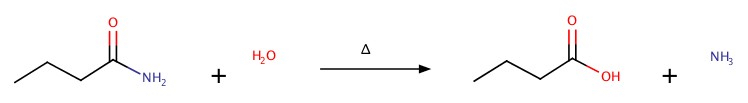
Amides
As we have seen amide has amine group next to C=O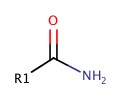
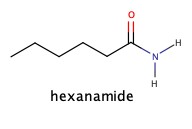
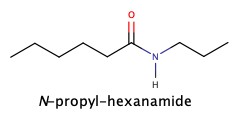
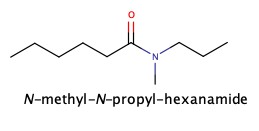
It can be synthesized by adding ammonia, NH3, to carboxylic acid:
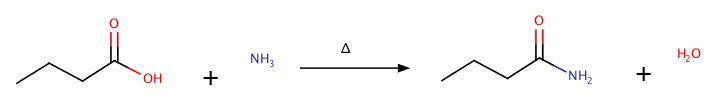
The reaction can be reversed by heating.
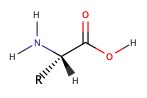
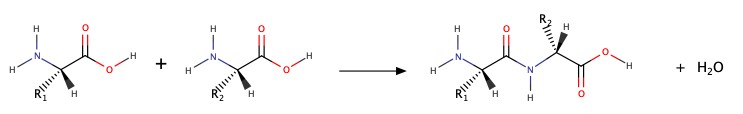
Summary of Rxns in Chapter 8
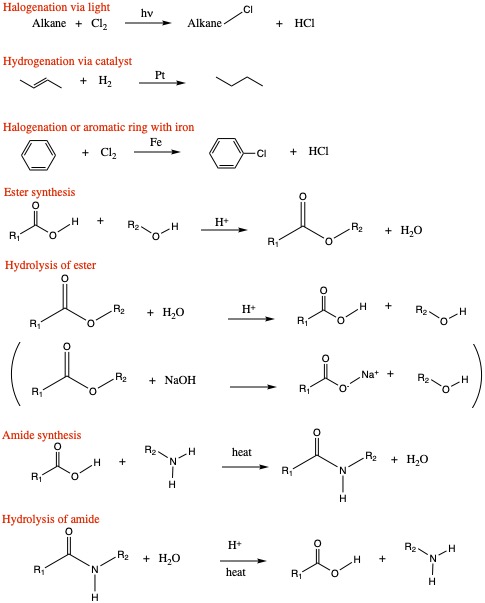
Additional Questions on Rxns