Chapter 1 Practice Problems
The practice problems are mainly to solve dimensional analysis and unit conversion problems. In all calculataions, we assume that you give correct significant figures at the end of the calculations. Please carry all digits until the end, then round.
None of trivial examples are included in this.
Example:1.85 The density of silver is 10.5 g/cm3. What is the mass in kg of a cube of silver that measures 0.62 m on each side?
The volume of the cube is (0.62 m)3. The question is how many kg are there in (0.62 m)3?
Example:1.52 Carry out the following conversions:
a. 5 pm = __ cm = __ nm
b. 8.5 cm3 = __ m3 = __ mm3
a. Ask, how many cm are there in 5 pm? Similarly,
b. Little tricky. But, the similar question, how many m3 are there in 8.5 cm3? Notice we're cubing the whole parenthesis!! Then, we can do the second part, as well.
Example:1.96 When an irregularly shaped chunk of silicon weighing 8.763 g was placed in a graduated cylinder containing 25.00 mL of water, the water level in the cylinder rose to 28.76 mL. What is the density of silicon in g/cm3?
Since The volume of silicon is given by the difference of the water levels before and after placing the silicon in, which is 3.76 mL. The conversion factor 1 mL = 1 cm3 is used in the last equality.
Example:1.105 Vinaigrette salad dressing consists mainly of oil and vinegar. The density of olive oil is 0.918 g/cm3, the density of vinegar is 1.006 g/cm3, and the two do not mix. If a certain mixture of olive oil and vinegar has a total mass of 397.8 g and a total volume of 422.8 cm3, what is the volume of oil and what is the volume of vninegar in the mixture?
Therefore, the volume of oil then is
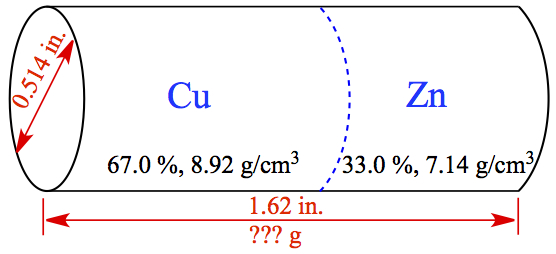
Example:1.112 Brass is a copper-zinc alloy. What is the mass in grams of a brass cylinder having a length of 1.62 in. and a diameter of 0.514 in. if the composition of the brass is 67.0 % copper and 33.0 % zinc by mass? The density of copper is 8.92 g/cm3, and the density of zinc is 7.14 g/cm3. Assume that the density of the brass varies linearly with composition.
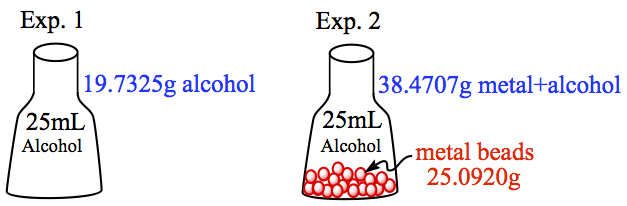
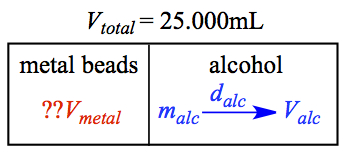
Example: A calibrated pycnometer with its volume 25.000 mL was filled with ethyl alcohol. By weighing the flask before and after adding the alcohol, it was determined that the pycnometer contains 19.7325 g of alcohol. In a second experiment, a 25.0920 g of metal beads were added to the pycnometer and the pycnometer was filled with alcohol to 25.000 mL mark. The total mass of the metal beads and alcohol in the pycnometer was deternined to be 38.4704 g. What is the density of the metal beads in g/mL?
Then, the density of metal beads is then
The following is from the back of chapter, and this is essentially the same as the previous question on picnometer.
Example:1.100. A graduated cylinder is filled to the 40.00-mL mark with a mineral oil. The masses of the cylinder before and after the addition of the oil are 124.966g and 159.446g, respectively. In a separate experiment, a metal ball (sphere) of mass 18.713g is placed in the cylinder and the cylinder is filled again to the 40.00-mL mark with the mineral oil. The combined mass of the ball and oil is 50.952g. Calculate the density and radius of the metal ball. The volume of sphere of radius r is (4/3)πr3.
This is nearly the same question as above! Do you see?
Example: What is the length of a 10-lb spool of 12 gauge aluminum wire? A 12 gauge has a diameter of 0.0808 in. and the density of aluminum is 2.70 g/cm3.
We can visualize the question by unfolding the spool and straighten the wire to a cylindrical form as follows.
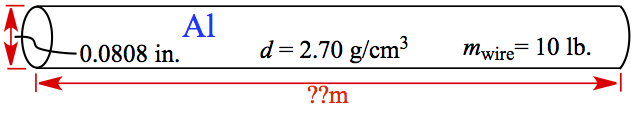
The volume of cylinder is given by where r is the radius of the circular face, and l is the length in question. Solving for l, we have Let's calculate the volume first. Since the length we want is in m, the volume we calculate should be in m3; so this is what we're looking for. What is our given? The given should be 10 lb. 12 gauge and 0.0808 in. are measures of diameter, which relates to r. So, only option is 10 lb. We ask, how many m3 are there in 10 lb.? For r we need the value in m. The radius of the circular area is 0.0404 in. So, Substitute the numbers into l = V /(πr2), we get.
Example: One acre-ft is defined as a volume of an acre of land filled with water to a depth of one foot. What is 1 acre-ft in ft3? and in m3, given that 640 acre = 1 mi2, 1 mi = 1.609344 km, 1 in. = 2.54 cm, and 12 in. = 1 ft?
The pictorial representation of the question is as follows.
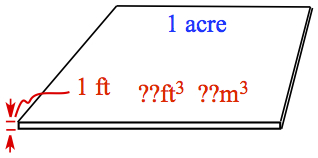
We want to know the volume of acre-ft in, first, ft3. If we convert the area of 1 acre in ft2, the volume is obtained by multiplying 1 ft at the end, so our question is, how many ft2 are there in 1 acre? The last arrow indicates that the height of 1 ft is multipled to get the volume.
Let's calculate now the volume in m3.
Example: Taken from the Prelab question: What is the volume of a room in L that hold 30.0 kg of oxygen (O2), if the density of O2 is 1.300 g/L, and oxygen in air is 21.0 % by volume?
The pictorial representation of the problem is
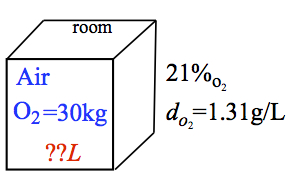
The key is to understand what each of the givens and what we are looking for. First, we are looking for the volume of the room. There seems to be no conversion factor that relates O2 to the room. But, air does relate to O2. So, instead of asking for the volume of the room, we are asking for the volume of air. Density is a conversion factor converting mass to volume or vise versa. The percent by volume is also a conversion factor because when expanded, it means that So, we use the ratio of 21 LO2 / 100 Lair as the conversion factor. It must mean that the 30.0 kg is our given, so that we ask, "How many L of air that contains a 30 kg of O2?" Then,
Example: 1.93 Sodium (Na) metal undergoes a chemical reaction with chlorine (Cl) gas to yield sodium chloride, table salt. If 1.00 g of sodium reacts with 1.54 g of chlorine, 2.54 g of sodium chloride is formed, 17.9 kJ of heat is released. How much sodium and how much chlorine in grams would have react to release 171 kcal of heat
Pictorially, you have
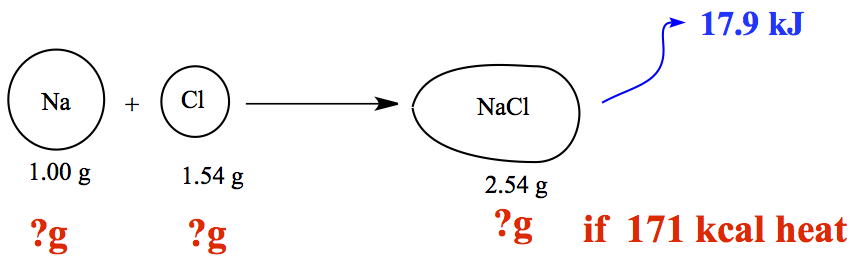
You can see that 17.9 kJ of heat generated per 1.00 g Na. So, we can use 17.9 kJ/gNa as a conversion factor. We ask,
Example: 1.92 Chlorine is used to disinfect swimming pools. The accepted concentration for this purpose is 1 ppm chlorine, or 1 g of chlorine per million g of water. Calculate the volume of a chlorine solution ( in mL) a homeowner should add to her swimming pool if the solution concentration is 6.0 % chlorine by mass and there are 2.0 x 104 gal of water in the pool. 1 gal = 3.79 L density of liquid = 1.0 g / mL