Chapter 4 Practice Problems
The practice problems are mainly to solve dimensional analysis and unit conversion problems. In all calculataions, we assume that you give correct significant figures at the end of the calculations. Please carry all digits until the end, then round.
None of trivial examples are included in this.
Example:How many g of PCl3 formed from 100 g of Cl2, if it was reacted with 50.0 g of P4 according to the following reaction? Also, how many g of P4 left after the reaction?
First, the chemical eqation is not balanced. So, you need to balance it.
In order for us to calculate the number of g of P4 left over, we need to know how many g of P4 used for producing 129 g of PCl3. So, we say,
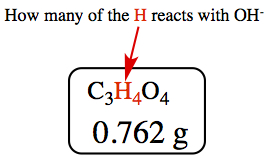
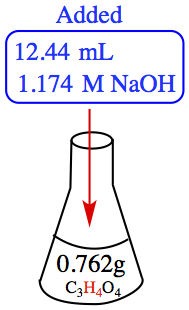
Example:1.120 The moecular formula of malonic acid is C3H4O4. If a solution containing 0.762 g of the acid is requires 12.44 mL of 1.174 M NaOH for neutralization, how many ionizable H atoms are present in the molecule?
The situation is that we can calculate the number of moles of H+ from the acid reacted by knowing the volume of NaOH with the number of H+ available for dissociation from the mass and the chemical formula.
Pictorially,
 |
 | |
| We can calculate the number of moles of H available in the molecule, depending on how many of the hydrogens dissociated because we know the molar mass! In principle (at the current chapter), you have up to four H available for dissociation. So, you might need to calculate for four different scenarios! | From this picture, we can calculate the number of moles of H consumued by the OH- ion. |
It means that we need to perform two different set of calculations: from the picture on left to see how many moles of H dissociates depending on the level of dissociation; you have up to four hydrogens, and from the picture on right to see how many moles of H actually dissociated. If the two calculations match in numbers you can tell how many of the H in the molcules dissociated.
If only one H dissociates:
If two H dissociate:
Just in case, if three H dissociate:
Now, the second part: We need to calculate the actual amount of H+ dissociated, for which it was neutralized by the OH- ion. For such calculation, you need to set up dimensional analysis. You ask yourself,
The [1.174 M] is the molarity, which contains two fundamental units mol and L or 103mL, thusly molarity is a conversion factor. So, we start our calculation by
The value just calculated corresponds to the two H dissoication. Therefore, there are two hydrogens in the molecule avialable for ionization.
Example:1.122 A 60.0 mL 0.513 M glucose (C6H12O6) solution is mixed with 120.0 mL of 2.33 M glucose solution. What is the final concentration, if you assume that the volume is additive?
Example: Calculate the concentration of chloride ions in the solution where 200.0 mL of 0.400M NaCl is titrated with the 80.0 mL of 0.750M AgNO3.
How many g of AgCl is produced by adding 160.0 mL of 0.75M AgNO3 into 200.0 mL of 0.400M NaCl?
Example:1.127 The molar mass of a certain metal carbonate, MCO3, can be determined by adding an excess of HCl acid to react with all the carbonate and then "back titrating" the remaining acid with a NaOH solution. (a) Write an equation for these reactions. (b) In a certain experiment, 20.00 mL of 0.0800 M HCl were added to a 0.1022 g sample of MCO3. The excess HCl required 5.64 mL of 0.1000 M NaOH for neutralization. Calculate the molar mass of the carbonate and identify M.
For simplification, we assume that the metal M of MCO3 molecule dissoicates into M2+ and CO32-. Then, it takes 2 mol H+ to make H2CO3.
The excess HCl part: Here the assumption is that the H2CO3 would not let any of H to dissociates.
The pictorial representation is:
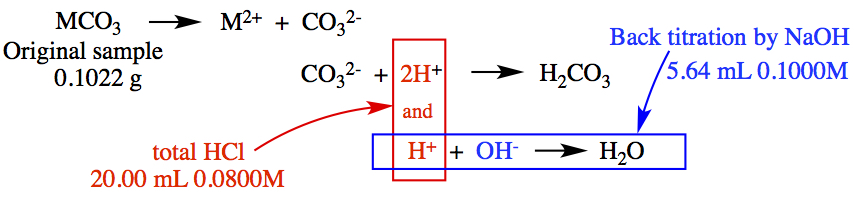
As you can see in the diagram above, there is a sequence of three reactions occur:
So, the strategy in solving this question is to obtain:
I'm not sure if the following is helpful, but it is pictorial representation of the strategy on how to solve the problem.
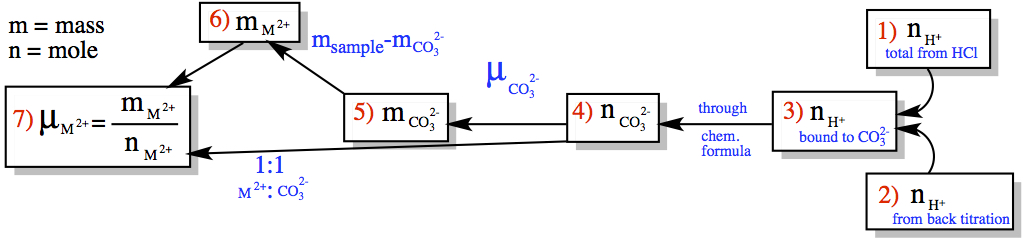
1) The total number of protons from the 0.0800 M HCl solution is 2) The amount of H+ used up by the back titration using 0.1000 M NaOH is 3) So, if we subtract the amount of H+ neutralized from the total amount of H+ came from HCl gives the amount of H+ bound to CO32-. 4) The number of moles of CO32- can be obtained from the second process in the diagram in 1:2 mole ratio of carbonate ion to hydrogen ion. 5) We can also obtain the number of g of CO32- as well, 6) This gives us the number of g of M2+ ion in the sample, 7) Then, the molar mass is calculated as
Looking up the Periodic Table reveals that the metal ion is Barium, Ba with its molar mass, 137.33 g/mol.
Example:1.128 A 5.012-g sample of an iron chloride hydrate was dried in an oven. The mass of the anhydrous compound was 3.195 g. The compound was then dissolved in water and reacted with an excess of AgNO3. The AgCl precipitate formed weighed 7.225 g. What is the formula of the original compound?
Example:1.131 The concentration of lead ions (Pb2+) in a sample of polluted water that also contains nitrate ions (NO3-) is determined by adding solid sodium sulfate (Na2SO4) to exactly 500 mL of the water. (a) Write the molecular and net ionic equations for the reaction. (b) Calculate the molar concentration of Pb2+ if 0.00450 g of Na2SO4 was needed for the complete precipitation of Pb2+ ions as PbSO4.
(a) The polluted sample contains Pb2+ and NO3-,and by adding Na2SO4 precipitates out PbSO4. Therefore, the molecular equation is:
There are spectator ions and are: Na+ and NO3- So they will be canceled out in the net ionic equation:
(b) The concentration of [Pb2+] is given in molarity by: for which the denominator is known, 500 mL or 0.5 L. It means that the question is really asking about the number of moles of Pb ion by using 0.00450 g of Na2SO4.
Then, the result is used to convert it into molarity unit,
Example:1.145 Acetylsalicylic acid (C9H8O4) is a monoprotic acid commonly known as "aspirin." A typical aspirin tablet, however, contains only a small amount of the acid. In an experiment to determine its composition, an aspirin tablet was crushed and dissolved in water. It took 12.25 mL of 0.1466 M NaOH to neutralize the solution. Calculate the number of grains of aspirin in the tablet. (One grain = 0.0648 g.)
It is presumed that the acetylsalicylic acid (weak acid) reacts with sodium hydroxide, the strong base, thus the net ionic equation is
The following question is related to the lab we have done.
Example:4.162 A quantity of 25.0 mL of a solution containing both Fe2+ and Fe3+ ions is titrated with 23.0 mL of 0.0200 M KMnO4 (in dilute sulfuric acid). As a result, all of the Fe2+ ions are oxidized to Fe3+ ions. Next, the solution is treated with Zn metal to convert all of the Fe3+ ions to Fe2+ ions. Finally, the solution containing only the Fe2+ ions requires 40.0 mL of the same KMnO4 solution for oxidation to Fe3+. Calculate the molar concentrations of Fe2+ and Fe3+ in the original solution. The net ionic equation is
So, we need to know the [Fe2+]original and [Fe3+]original, both of which we do know the volume, 25.0 mL.
The first titration give the Fe2+ in the original solution.
The second titration gives the total amount of Fe in the solution. By this
time, all Fe ions are converted to Fe2+. Thus, by calulating the
amount of Fe2+ in the second titration and its difference with
the first one gives the [Fe3+]original.
Example: 2.111 A sample of naturally occuring silicon consists of 28Si (27.9869 amu), 29Si (28.9765 amu) and 30Si (29.9738 amu). If the atomic mass of silicon is 28.0855 and the natural abundance of 29Si is 4.68 %, what are the natural abundances of 28Si and 30Si?
This is solved exactly the same way as we did in the class for copper. The key equation is for which we know < m >, all masses of isotopes, and c29. So, we have an equation with two unknowns. We need one more relation. We have for natural abundance in fractional form, We rearrange above to solve for c28, we get Substitute this into the first equation, we have Isolate c30, Put numbers in which gives c30 = 0.073115728 = 0.0731 or 7.31%. Then, c28 is 100% - 4.68 - 7.31 = 88.0%.
Example: Calcium levels in blood can be determined by adding oxalate ion to precipitate calcium oxalate, CaC2O4, followed by dissolving the precipitate in aqueous acid and titrating the resulting oxalic acid, H2C2O4, with KMnO4:
Example: Iodine, I2, reacts with aqueous thiosulfate ion in neutral solution according to the balanced equation